Properties of Solids Commonly Observed Properties of Solids

Properties of Solids

Commonly Observed Properties of Solids 1) Have definite shape and volume; rigid and shapes are fixed 2) Do not easily compress or expand Temperature & pressure have negligible effects on the volume of solids 3) Have higher densities than liquids. 4) Do not mix by diffusion. 5) Either crystalline or non crystalline in form.
TERMS TO REMEMBER CRYSTALLOGRAPHY – science that explains the definite geometric forms of crystals LATTICE – a three dimensional arrangement of points that represents sites with identical surroundings in the same orientation. UNIT CELL – fundamental unit of the crystal lattice. ALLOTROPES – crystalline solids that exist in various forms

TWO TYPES OF SOLID STRUCTURES I Crystalline solid II Amorphous solid
CRYSTALLINE SOLIDS • Consists of regularly repeated, organized patterns of molecules • Have definite melting points • Symmetry of crystals can be describe in terms of crystal lattice. • Crystals can be ionic, molecular or metallic
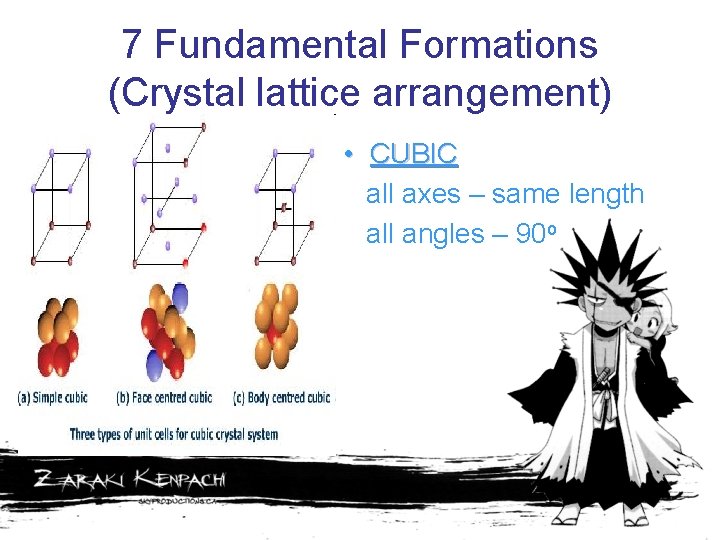
7 Fundamental Formations (Crystal lattice arrangement) • CUBIC all axes – same length all angles – 90 o
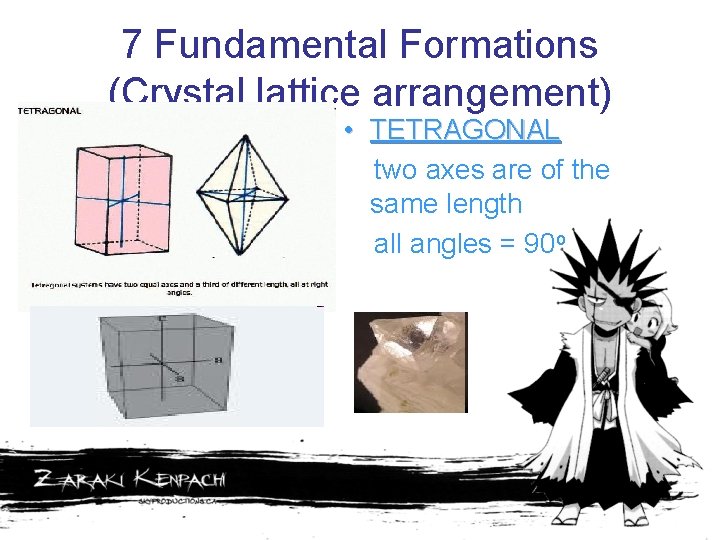
7 Fundamental Formations (Crystal lattice arrangement) • TETRAGONAL two axes are of the same length all angles = 90 o
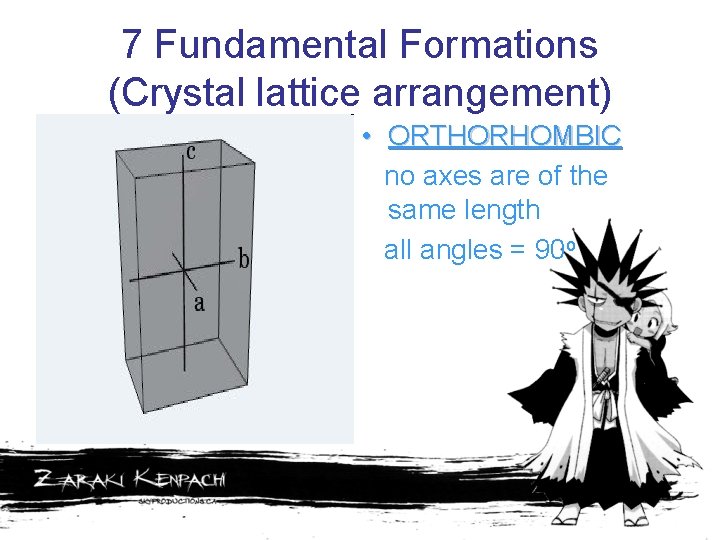
7 Fundamental Formations (Crystal lattice arrangement) • ORTHORHOMBIC no axes are of the same length all angles = 90 o
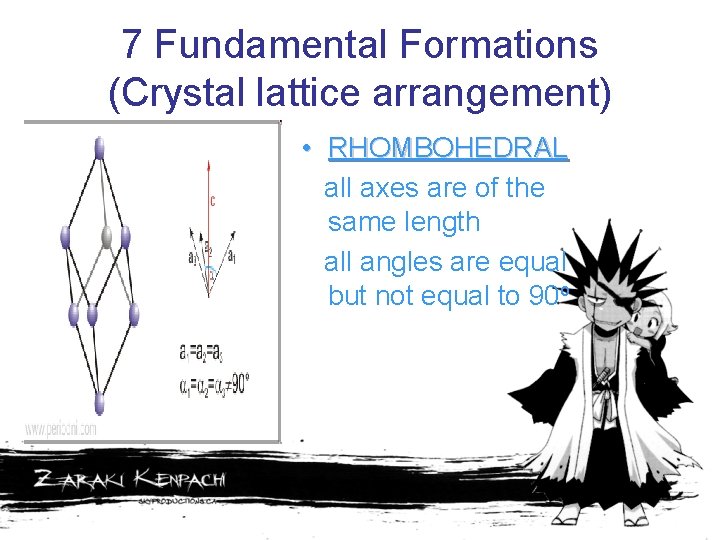
7 Fundamental Formations (Crystal lattice arrangement) • RHOMBOHEDRAL all axes are of the same length all angles are equal but not equal to 90 o

7 Fundamental Formations (Crystal lattice arrangement) • MONOCLINIC no axes are of the same length two angles = 90 o
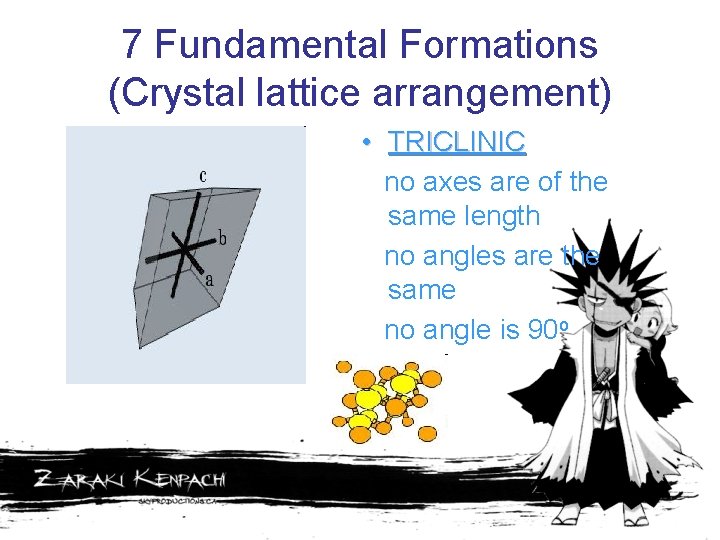
7 Fundamental Formations (Crystal lattice arrangement) • TRICLINIC no axes are of the same length no angles are the same no angle is 90 o
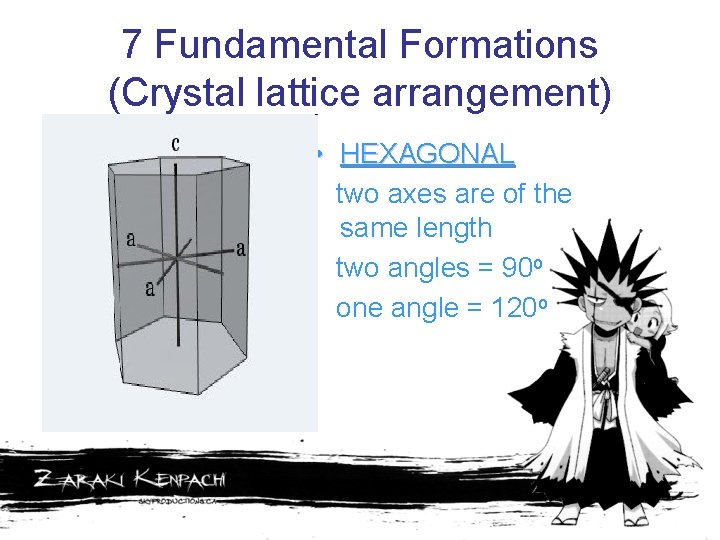
7 Fundamental Formations (Crystal lattice arrangement) • HEXAGONAL two axes are of the same length two angles = 90 o one angle = 120 o
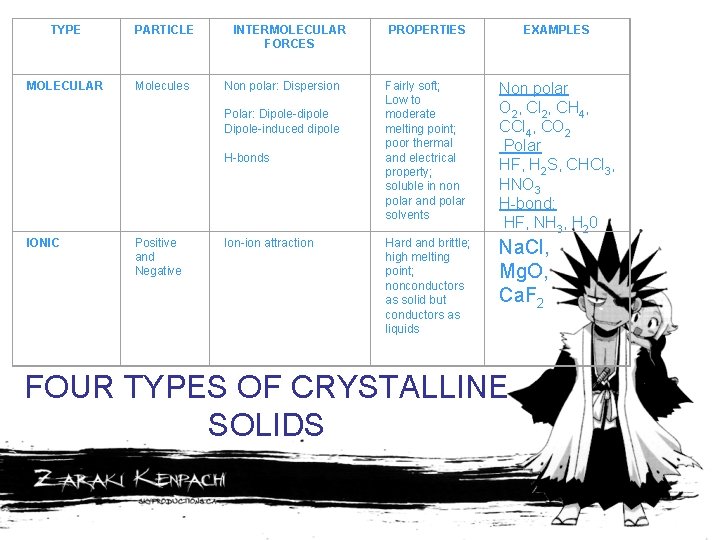
TYPE PARTICLE MOLECULAR Molecules INTERMOLECULAR FORCES Non polar: Dispersion Polar: Dipole-dipole Dipole-induced dipole H-bonds IONIC Positive and Negative Ion-ion attraction PROPERTIES EXAMPLES Fairly soft; Low to moderate melting point; poor thermal and electrical property; soluble in non polar and polar solvents Non polar O 2, Cl 2, CH 4, CCl 4, CO 2 Polar HF, H 2 S, CHCl 3, HNO 3 H-bond: HF, NH 3, H 20 Hard and brittle; high melting point; nonconductors as solid but conductors as liquids Na. Cl, Mg. O, Ca. F 2 FOUR TYPES OF CRYSTALLINE SOLIDS

TYPE PARTICLE INTERMOLECULAR FORCES PROPERTIES EXAMPLES COVALENT NETWORK atoms Covalent bond Very hard; very high melting point; poor thermal and electrical property C (diamond) C (graphite) Si. O 2 METALLIC cations and delocalized electron Metallic bond soft to hard; low to high melting point; good thermal and electrical property; malleable and ductile Na, Mg, Fe, Cu, Zn, Hg, Pb FOUR TYPES OF CRYSTALLINE SOLIDS

AMORPHOUS SOLIDS • Have no regular arrangement of their molecules • Appear like an instantaneous photo of a liquid • Non crystalline • Lack the definite melting point • When heated, it gets softer and softer • Lack any preferential planes or edges • Ex. glass, rubber, plastics

Liquid Crystals • Phase of matter whose order is intermediate between that of a liquid and that of a crystalline solid. • Molecular arrangement is random in some direction and ordered in others • Consists of individual molecules (long & cylindrical) • Allows intermolecular attraction but inhibits cyrstalline packing. • Temperature sensitive (cold-solid; hot-liquid)
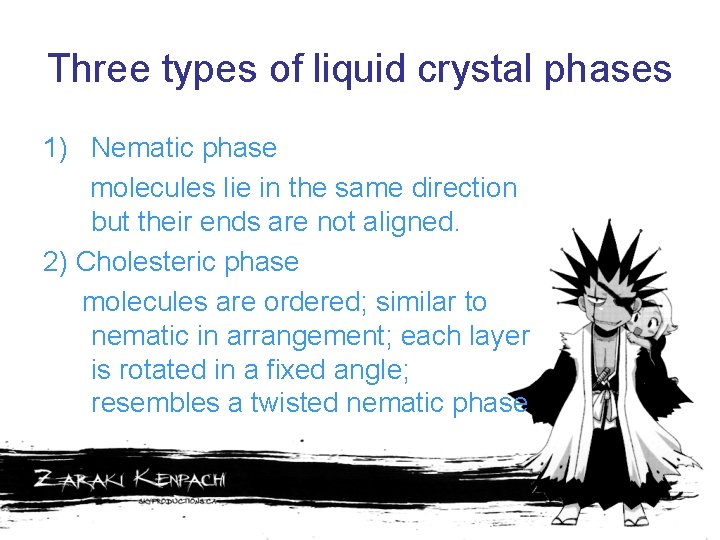
Three types of liquid crystal phases 1) Nematic phase molecules lie in the same direction but their ends are not aligned. 2) Cholesteric phase molecules are ordered; similar to nematic in arrangement; each layer is rotated in a fixed angle; resembles a twisted nematic phase

Three types of liquid crystal phases 3) Smectic phase it is close to the solid phase liquid crystals are ordered in layers (inside layers, liquid crystals normally float around freely) The ability of molecules in liquid crystals to realign/ Re-orient allows scientists to produce materials of great Strength and unique optical ability. Ex. sporting equipment, supersonic crafts, LCDs

THE END GOOD LUCK!!! STUDY HARD FOR YOUR QUARTERLY EXAMS!
- Slides: 19