Properties of Solids Classifying Solids can be classified

Properties of Solids

Classifying Solids can be classified according to 1. Arrangement of particles: Amorphous solids – particles arrangements lack order (rubber & glass) Crystalline solids – with organized particle arrangements and as a result a distinct shape 2. Bonds that hold them together (i. e. Ionic, covalent)

Properties of Solids • • Properties of solids depend on the forces between particles 4 types of solids 1. 2. 3. 4. Ionic (cation and anion) Metallic (metals) Molecular (nonmetals) Covalent Network (metalloid/carbon)
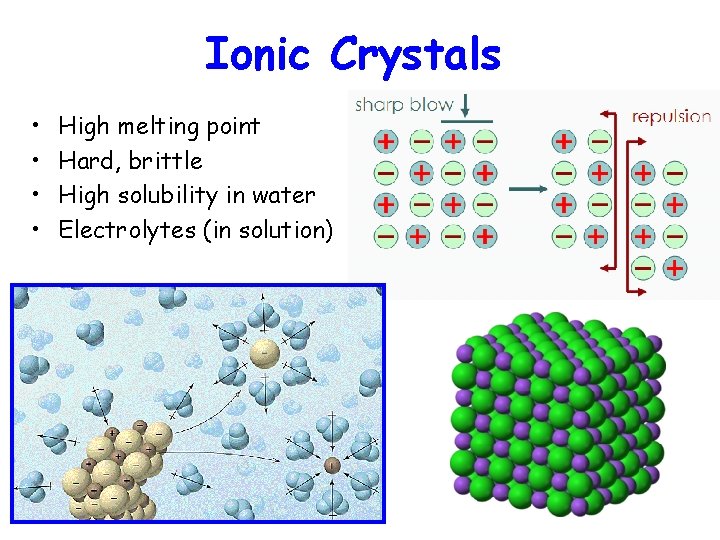
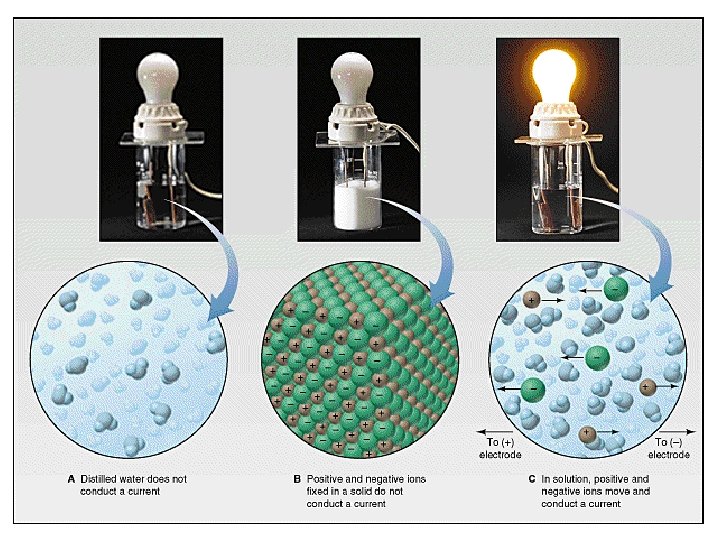
Ionic Crystals • • High melting point Hard, brittle High solubility in water Electrolytes (in solution)
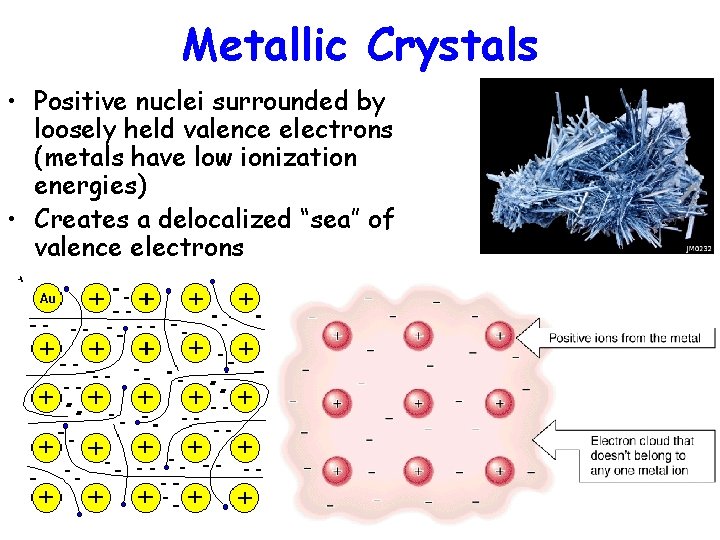
Metallic Crystals • Positive nuclei surrounded by loosely held valence electrons (metals have low ionization energies) • Creates a delocalized “sea” of valence electrons
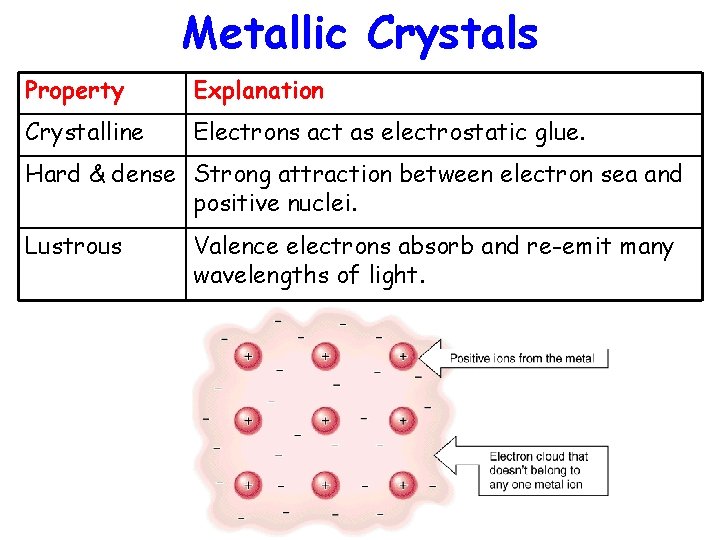
Metallic Crystals Property Explanation Crystalline Electrons act as electrostatic glue. Hard & dense Strong attraction between electron sea and positive nuclei. Lustrous Valence electrons absorb and re-emit many wavelengths of light.
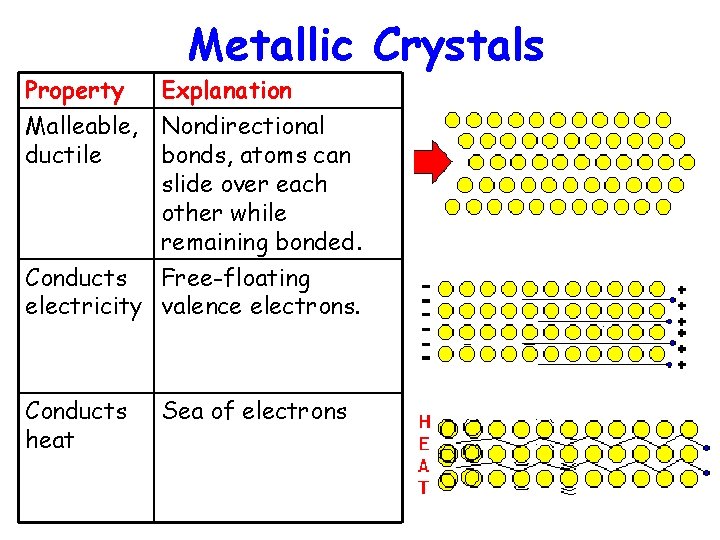
Metallic Crystals Property Explanation Malleable, Nondirectional ductile bonds, atoms can slide over each other while remaining bonded. Conducts Free-floating electricity valence electrons. Conducts heat Sea of electrons

Molecular Crystals • Soft • Low melting point • Nonelectrolytes

Covalent Network Crystals • Very hard and brittle • Very high melting points • Nonelectrolytes
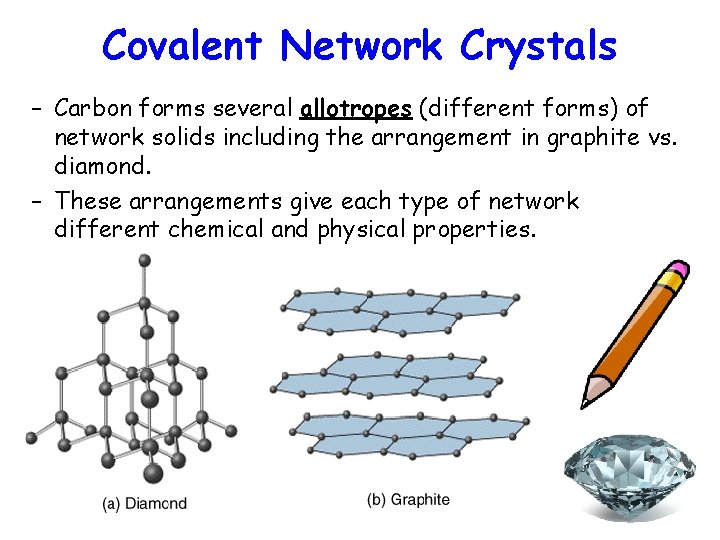
Covalent Network Crystals – Carbon forms several allotropes (different forms) of network solids including the arrangement in graphite vs. diamond. – These arrangements give each type of network different chemical and physical properties.

Practice • • Unknown Solids Worksheet Solids Research Worksheet P. 273 #1 -5, 7 P. 276 #1 -8
- Slides: 12