Properties of seawater Properties of water 1 Polarity








- Slides: 15

Properties of seawater

Properties of water 1. Polarity and hydrogen bonding cohesion good solvent l many molecules dissolve in H 2 O 2. lower density as a solid l ice floats! 3. high heat capacity l l water stores heats & cools slowly

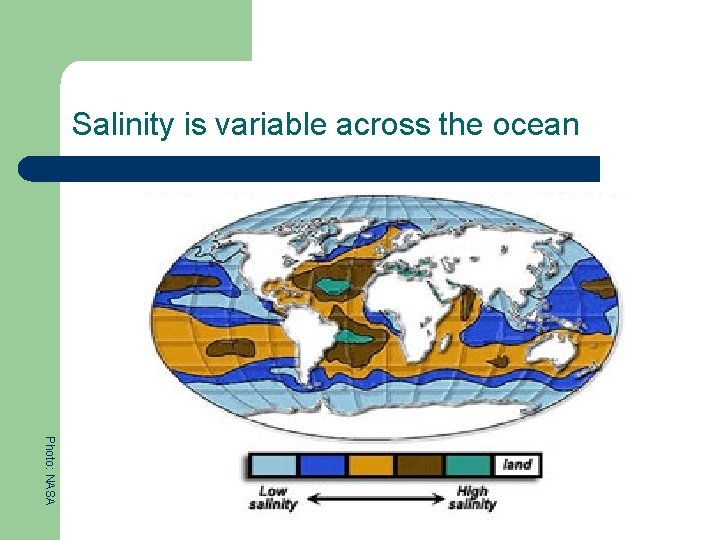
Salinity is an important part of ocean chemistry 1. 2. 3. Salinity is a measure of the amount of dissolved salts in water Salinity is not homogenous (uniform) across the Earth’s oceans Both salinity and temperature affect the density of seawater

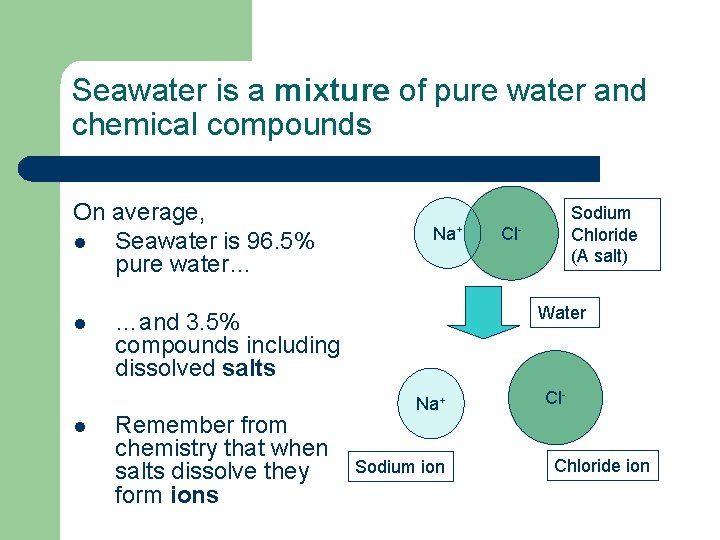
Seawater is a mixture of pure water and chemical compounds On average, l Seawater is 96. 5% pure water… l l Na+ Cl- Water …and 3. 5% compounds including dissolved salts Remember from chemistry that when salts dissolve they form ions Sodium Chloride (A salt) Na+ Sodium ion Cl- Chloride ion

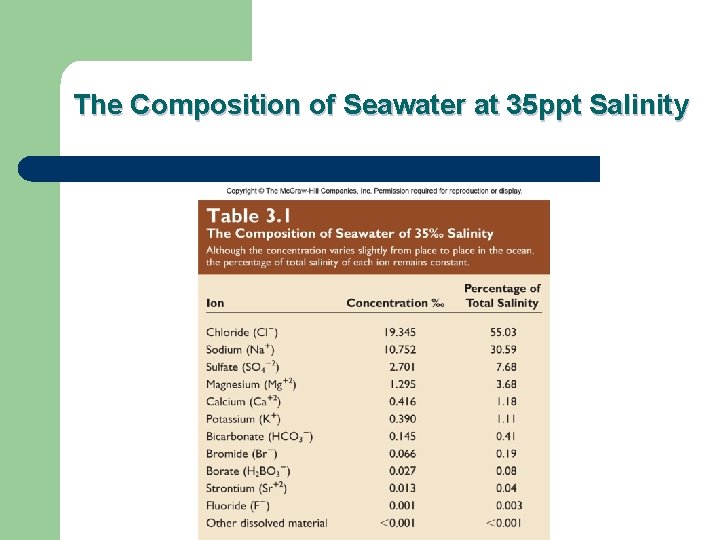
What’s in the water? l 7 primary chemicals make up almost all (~99%) the salts in seawater: – – – – l Chloride (Cl-): 55% Sodium (Na+): 30. 6% Sulfate (SO 42 -): 7. 7% Magnesium (Mg 2+): 3. 7% Calcium (Ca 2+): 1. 2% Potassium (K+): 1. 1% Bicarbonate (HCO 3 -): 0. 4% Can you come up with an acronym to remember them all?

How do scientists figure out how much salt is in the water? l The Rule of Constant Proportions = the major ions of seawater are present in a fixed proportion to each other – – This means that although salinity may vary, the ratio of any one of the 7 primary ocean salts to each other will not change If we have a liter of seawater that has a 35 grams of total salt per liter, how many grams of calcium are there?

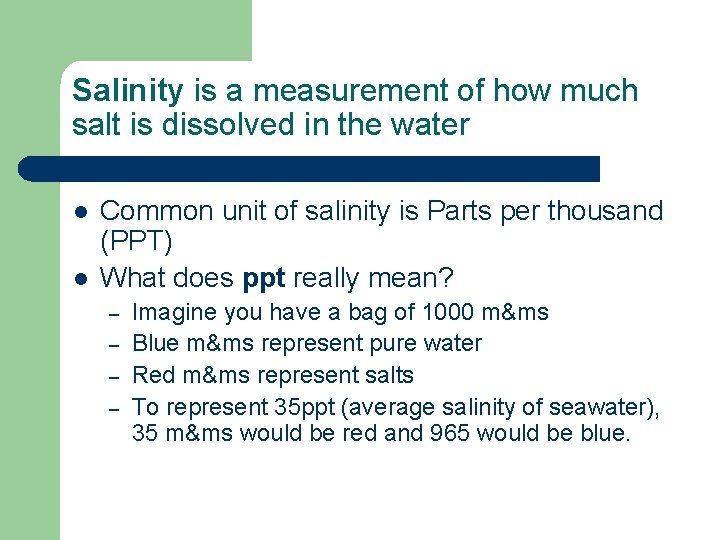
Salinity is a measurement of how much salt is dissolved in the water l l Common unit of salinity is Parts per thousand (PPT) What does ppt really mean? – – Imagine you have a bag of 1000 m&ms Blue m&ms represent pure water Red m&ms represent salts To represent 35 ppt (average salinity of seawater), 35 m&ms would be red and 965 would be blue.

The Composition of Seawater at 35 ppt Salinity

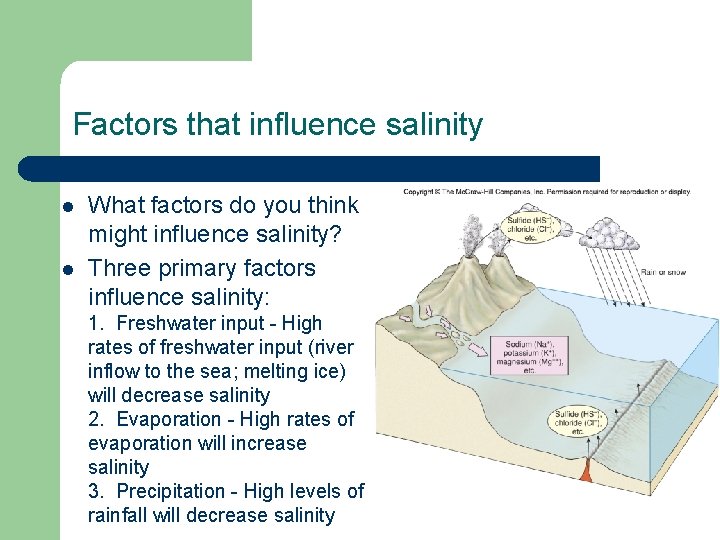
Factors that influence salinity l l What factors do you think might influence salinity? Three primary factors influence salinity: 1. Freshwater input - High rates of freshwater input (river inflow to the sea; melting ice) will decrease salinity 2. Evaporation - High rates of evaporation will increase salinity 3. Precipitation - High levels of rainfall will decrease salinity

Salinity is variable across the ocean Photo: NASA

Why is salinity important? l l Salinity is one factor that controls the density of ocean water What happens when water at different depths has different densities? – – Layers of water will form Formation of layers is part of the reason we have ocean currents

Gases in Seawater l Many gases are also dissolved in seawater including oxygen, carbon dioxide, and nitrogen l Gases dissolve at the sea surface from the atmosphere (gas exchange) and vice versa. l Gases dissolve better in cold water than in warm.

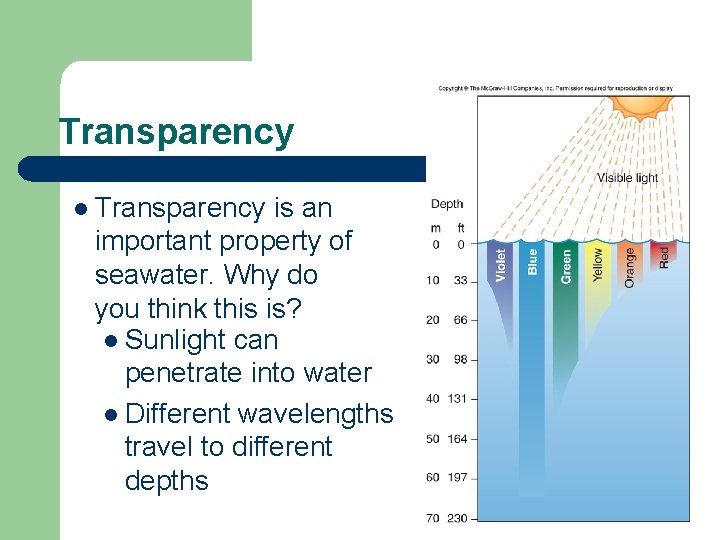
Transparency l Transparency is an important property of seawater. Why do you think this is? l Sunlight can penetrate into water l Different wavelengths travel to different depths

Conditions vary with depth l What do you think happens to each of the following with increasing depth? – – – Oxygen Carbon dioxide Temperature Light Pressure

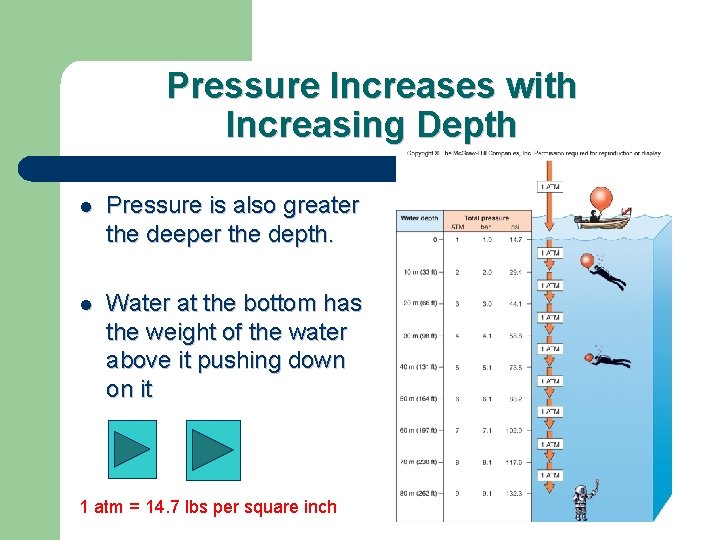
Pressure Increases with Increasing Depth l Pressure is also greater the deeper the depth. l Water at the bottom has the weight of the water above it pushing down on it 1 atm = 14. 7 lbs per square inch