PROPERTIES OF PERIOD 3 ELEMENTS Learning Objectives 1

PROPERTIES OF PERIOD 3 ELEMENTS Learning Objectives: 1. Identify the products of the reactions of sodium and magnesium with water. 2. Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present. 3. Explain trends in the melting points of period 3 oxides in terms of structure and bonding. Specification Reference: 3. 2. 4
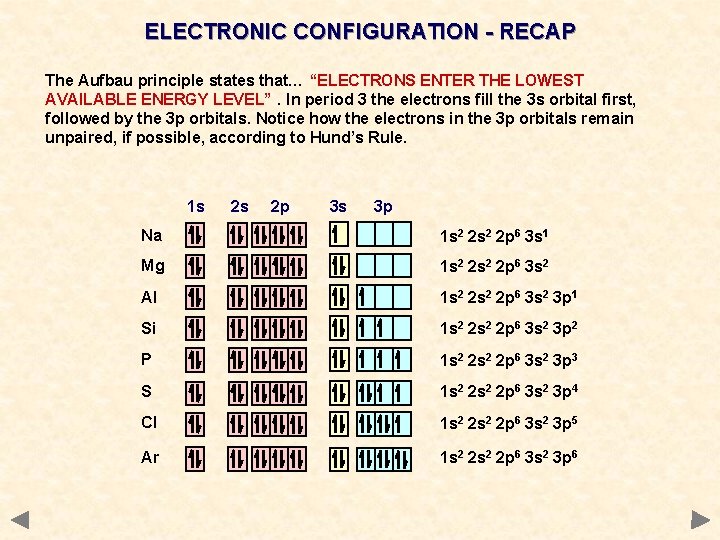
ELECTRONIC CONFIGURATION - RECAP The Aufbau principle states that… “ELECTRONS ENTER THE LOWEST AVAILABLE ENERGY LEVEL”. In period 3 the electrons fill the 3 s orbital first, followed by the 3 p orbitals. Notice how the electrons in the 3 p orbitals remain unpaired, if possible, according to Hund’s Rule. 1 s 2 s 2 p 3 s 3 p Na 1 s 2 2 p 6 3 s 1 Mg 1 s 2 2 p 6 3 s 2 Al 1 s 2 2 p 6 3 s 2 3 p 1 Si 1 s 2 2 p 6 3 s 2 3 p 2 P 1 s 2 2 p 6 3 s 2 3 p 3 S 1 s 2 2 p 6 3 s 2 3 p 4 Cl 1 s 2 2 p 6 3 s 2 3 p 5 Ar 1 s 2 2 p 6 3 s 2 3 p 6
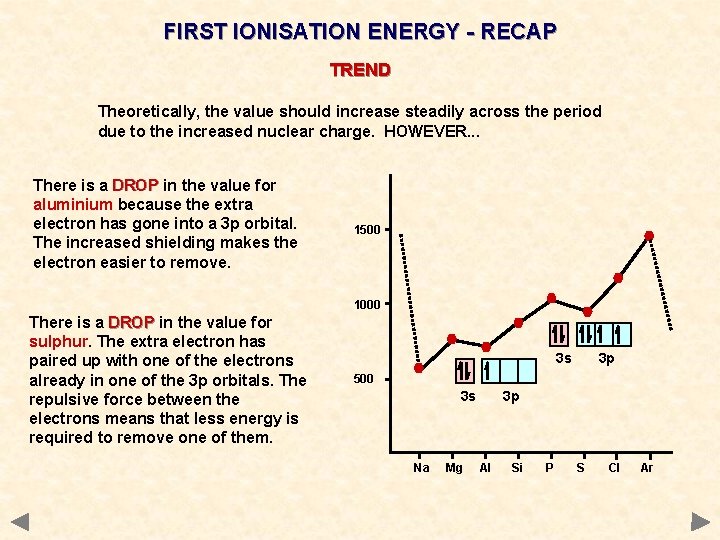
FIRST IONISATION ENERGY - RECAP TREND Theoretically, the value should increase steadily across the period due to the increased nuclear charge. HOWEVER. . . There is a DROP in the value for aluminium because the extra electron has gone into a 3 p orbital. The increased shielding makes the electron easier to remove. 1500 1000 There is a DROP in the value for sulphur. The extra electron has paired up with one of the electrons already in one of the 3 p orbitals. The repulsive force between the electrons means that less energy is required to remove one of them. 3 s 3 p 500 3 s Na Mg 3 p Al Si P S Cl Ar

METALS OF PERIOD 3 REACTION WITH WATER Sodium • vigorous reaction • hydrogen evolved • strong alkaline solution produced (p. H = 14) Na(s) + 2 H 2 O(l) Magnesium ——> 2 Na. OH(aq) + H 2(g) • slow reaction with cold water • weaker alkaline solution produced (p. H 9 -11) Mg(s) + 2 H 2 O(l) ——> Mg(OH)2(aq) + H 2(g) • very fast reaction with steam Mg(s) + H 2 O(l) ——> Mg. O(S) + H 2(g) LO 1: Identify the products of the reactions of sodium and magnesium with water.
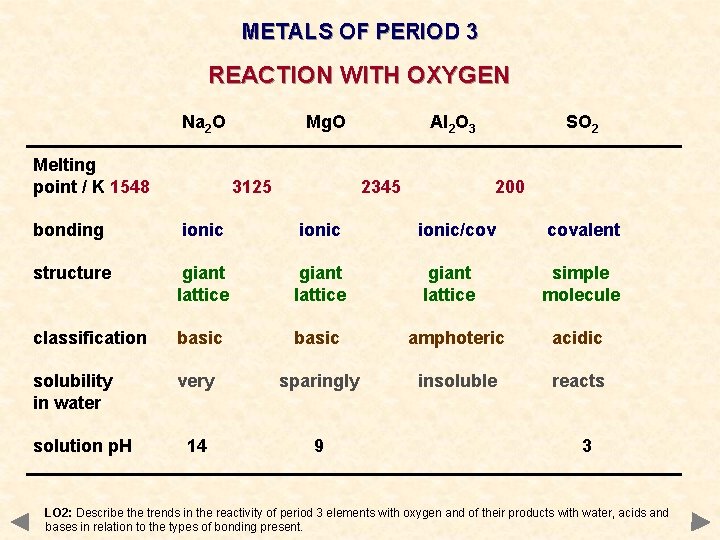
METALS OF PERIOD 3 REACTION WITH OXYGEN Na 2 O Melting point / K 1548 Mg. O 3125 Al 2 O 3 2345 SO 2 200 bonding ionic/cov covalent structure giant lattice simple molecule classification basic amphoteric acidic solubility in water very sparingly insoluble reacts 14 9 solution p. H 3 LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
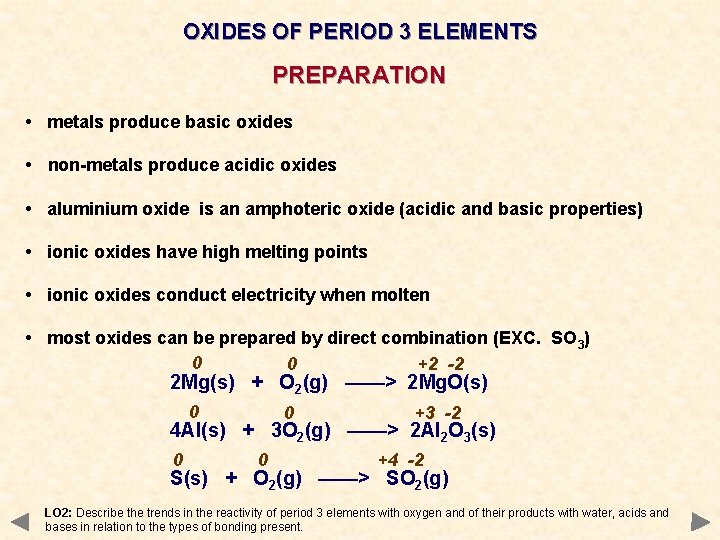
OXIDES OF PERIOD 3 ELEMENTS PREPARATION • metals produce basic oxides • non-metals produce acidic oxides • aluminium oxide is an amphoteric oxide (acidic and basic properties) • ionic oxides have high melting points • ionic oxides conduct electricity when molten • most oxides can be prepared by direct combination (EXC. SO 3) 0 0 +2 -2 2 Mg(s) + O 2(g) ——> 2 Mg. O(s) 0 0 +3 -2 4 Al(s) + 3 O 2(g) ——> 2 Al 2 O 3(s) 0 0 +4 -2 S(s) + O 2(g) ——> SO 2(g) LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
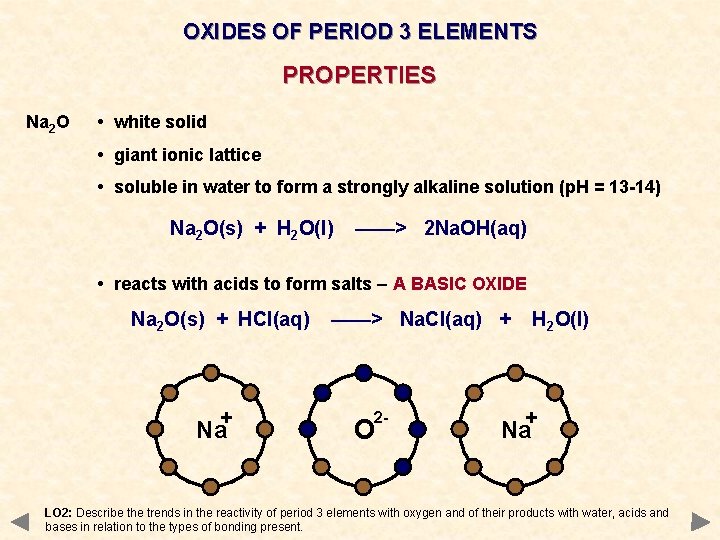
OXIDES OF PERIOD 3 ELEMENTS PROPERTIES Na 2 O • white solid • giant ionic lattice • soluble in water to form a strongly alkaline solution (p. H = 13 -14) Na 2 O(s) + H 2 O(l) ——> 2 Na. OH(aq) • reacts with acids to form salts – A BASIC OXIDE Na 2 O(s) + HCl(aq) + Na ——> Na. Cl(aq) + 2 - O H 2 O(l) + Na LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
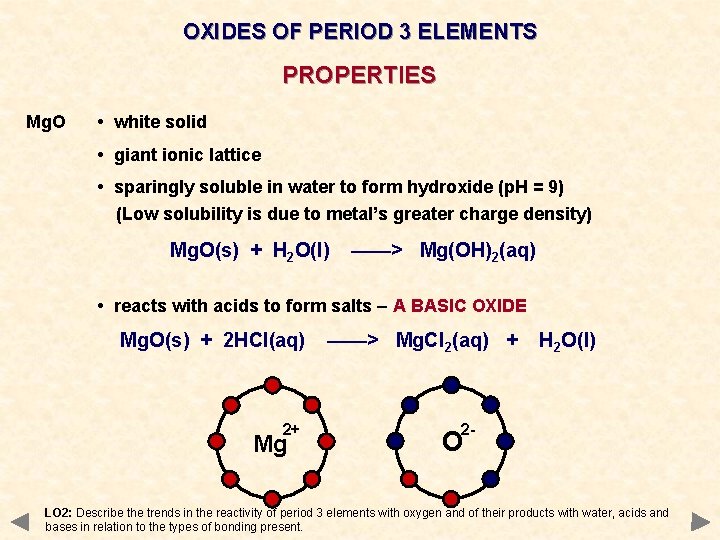
OXIDES OF PERIOD 3 ELEMENTS PROPERTIES Mg. O • white solid • giant ionic lattice • sparingly soluble in water to form hydroxide (p. H = 9) (Low solubility is due to metal’s greater charge density) Mg. O(s) + H 2 O(l) ——> Mg(OH)2(aq) • reacts with acids to form salts – A BASIC OXIDE Mg. O(s) + 2 HCl(aq) 2+ Mg ——> Mg. Cl 2(aq) + H 2 O(l) 2 - O LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
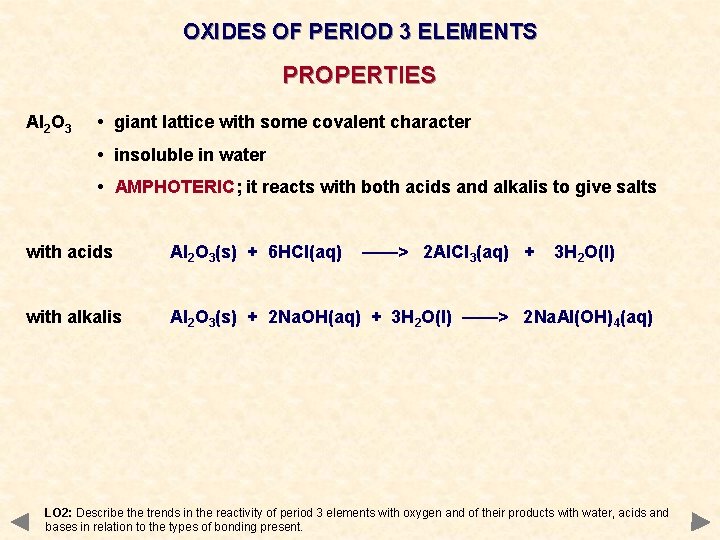
OXIDES OF PERIOD 3 ELEMENTS PROPERTIES Al 2 O 3 • giant lattice with some covalent character • insoluble in water • AMPHOTERIC; it reacts with both acids and alkalis to give salts with acids Al 2 O 3(s) + 6 HCl(aq) ——> 2 Al. Cl 3(aq) + 3 H 2 O(l) with alkalis Al 2 O 3(s) + 2 Na. OH(aq) + 3 H 2 O(l) ——> 2 Na. Al(OH)4(aq) LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
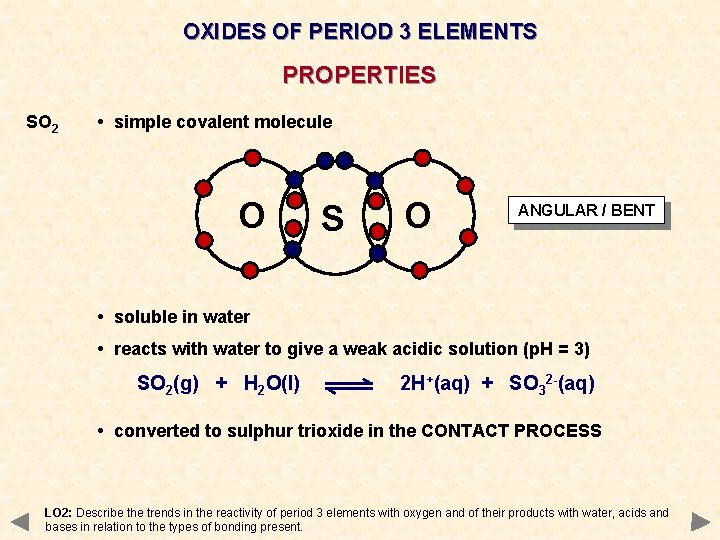
OXIDES OF PERIOD 3 ELEMENTS PROPERTIES SO 2 • simple covalent molecule O S O ANGULAR / BENT • soluble in water • reacts with water to give a weak acidic solution (p. H = 3) SO 2(g) + H 2 O(l) 2 H+(aq) + SO 32 -(aq) • converted to sulphur trioxide in the CONTACT PROCESS LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
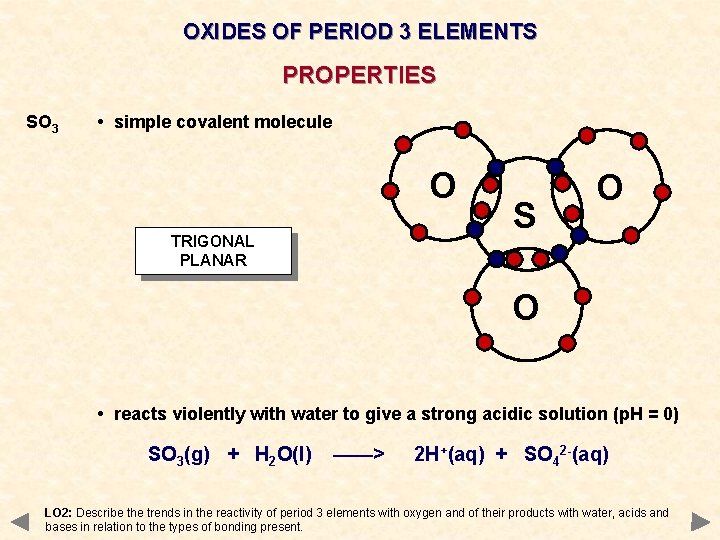
OXIDES OF PERIOD 3 ELEMENTS PROPERTIES SO 3 • simple covalent molecule O TRIGONAL PLANAR S O O • reacts violently with water to give a strong acidic solution (p. H = 0) SO 3(g) + H 2 O(l) ——> 2 H+(aq) + SO 42 -(aq) LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
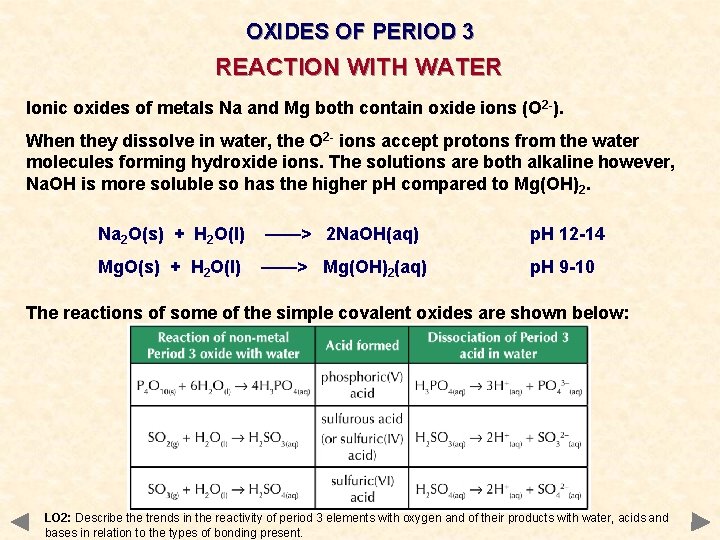
OXIDES OF PERIOD 3 REACTION WITH WATER Ionic oxides of metals Na and Mg both contain oxide ions (O 2 -). When they dissolve in water, the O 2 - ions accept protons from the water molecules forming hydroxide ions. The solutions are both alkaline however, Na. OH is more soluble so has the higher p. H compared to Mg(OH) 2. Na 2 O(s) + H 2 O(l) ——> 2 Na. OH(aq) p. H 12 -14 Mg. O(s) + H 2 O(l) ——> Mg(OH)2(aq) p. H 9 -10 The reactions of some of the simple covalent oxides are shown below: LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.

OXIDES OF PERIOD 3 REACTIONS WITH ACIDS AND BASES The equation for neutralising an acid and base is: Sodium and Magnesium oxides are basic so will neutralise acids: Na 2 O(s) + 2 HCl(aq) ——> 2 Na. Cl + H 2 O(l) Mg. O(s) + H 2 SO 4(aq) ——> Mg. SO 4(aq) + H 2 O(l) Silicon, Phosphorous and Sulphur oxides are basic so will neutralise bases: Si. O 2(s) + 2 Na. OH(aq) ——> Na 2 Si. O 3(aq) + H 2 O(l) P 4 O 10(s) + 12 Na. OH(aq) ——> 4 Na 3 PO 4(aq) + 6 H 2 O(l) SO 2(g) + 2 Na. OH(aq) ——> Na 2 SO 3(aq) + H 2 O(l) SO 3(g) + 2 Na. OH(aq) ——> Na 2 SO 4(aq) + H 2 O(l) Aluminium oxides are amphoteric so will neutralise acids or bases: Al 2 O 3(s) + 3 H 2 SO 4(aq) ——> Al 2(SO 4)3(aq) + 3 H 2 O(l) Al 2 O 3(s) + 2 Na. OH(aq) ——> 3 H 2 O(l) + 2 Na. Al(OH)4(aq) LO 2: Describe the trends in the reactivity of period 3 elements with oxygen and of their products with water, acids and bases in relation to the types of bonding present.
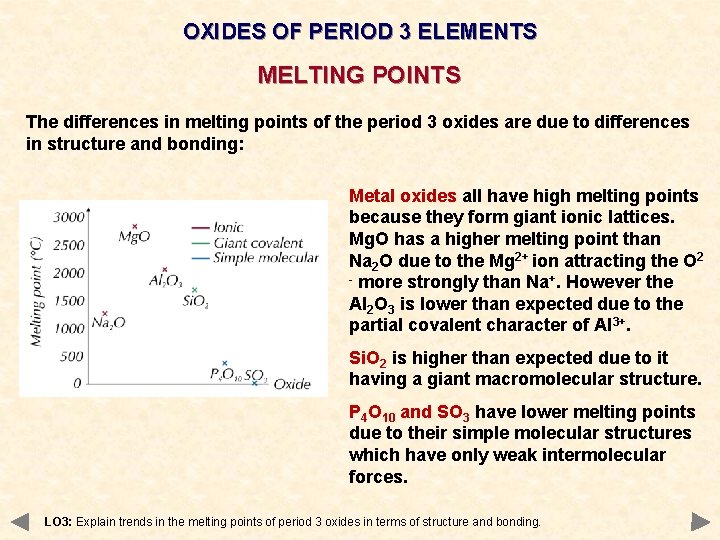
OXIDES OF PERIOD 3 ELEMENTS MELTING POINTS The differences in melting points of the period 3 oxides are due to differences in structure and bonding: Metal oxides all have high melting points because they form giant ionic lattices. Mg. O has a higher melting point than Na 2 O due to the Mg 2+ ion attracting the O 2 - more strongly than Na+. However the Al 2 O 3 is lower than expected due to the partial covalent character of Al 3+. Si. O 2 is higher than expected due to it having a giant macromolecular structure. P 4 O 10 and SO 3 have lower melting points due to their simple molecular structures which have only weak intermolecular forces. LO 3: Explain trends in the melting points of period 3 oxides in terms of structure and bonding.
- Slides: 14