PROPERTIES OF MATTER What is matter Anything that




















- Slides: 24

PROPERTIES OF MATTER

What is matter? ■ Anything that has mass and takes up space ■ Examples: water, air, people, animals, plants, bacteria, rocks, soil, salt

Mass versus weight ■ Mass: – The amount of matter in an object. – Does not change. ■ Weight – How much gravity is pulling down on the mass of an object. – Changes based on gravitational pull.

What is volume? ■ The amount of space an object takes up (occupies) ■ It is measured in different ways: 1) Formula (length x width x height) 2) Water displacement for irregular shapes 3) Graduated cylinder for liquids

Units of Volume ■ Volume is measured as a liter (L) or milliliter (m. L) or cubic centimeter (cm 3) ■ 1 m. L = 1 cm 3

What is density? ■ Density measures the amount of mass in a given volume – Density does not change based on the amount of a material. – Density is a property of that material.

How do you find density? ■ First, you need to know the objects mass (m) in grams and volume (v) in m. L ■ Then you can use this formula to figure out the density: Density = mass / volume The units for density are: grams / m. L (how it is expressed)

Density of Water Density of water = 1 g / m. L or 1 g / cm 3 If an objects density is greater that the density of water, then it will sink. If an objects density is less than water, it will float.

Physical Properties of Matter ■ A characteristic of a substance that can be observed and measured without changing the material. ■ Your senses are used to detect physical properties. ■ What is it called when you use your 5 senses to detect something?

Qualitative vs. Quantitative Observation ■ What is qualitative observation? – Making a descriptive observation about something ■ What is quantitative observation? – Making an observation about something that uses a measurement (numbers)

Physical Properties of Matter ■ The following are common physical properties of matter: – Density – Electrical conductivity – Heat conductivity – Solubility – Malleability – Magnetism – Melting point – Boiling point http: //studyjams. scholastic. com/studyjams/science/matter/properties-of-

Density Mass and Volume ■ Mass is the amount of matter in an object measured in grams – If you change the mass of a material, it will still be that material ■ Volume is the amount of space that a material takes up -If you change the volume of a material, it will still be that same material

Physical Properties of Matter – Electrical Conductivity ■ How well an electrical current moves through something. ■ The ability for an electrical current to move through something does not change based on the amount of that material ■ If a material does not conduct electricity it is known as an insulator https: //www. bing. com/videos/search? q=electrical+conducti vity&&view=detail&mid=7111 D 7 A 21 B 96 C 015 FA 4 E&&FORM=VRDGAR

Physical Properties of Matter – Heat Conductivity ■ How well heat moves through something. ■ The ability for heat to move through something does not change based on the amount of that material https: //www. youtube. com/watch? v=Ry 8 y. Xh. Cxcl. A

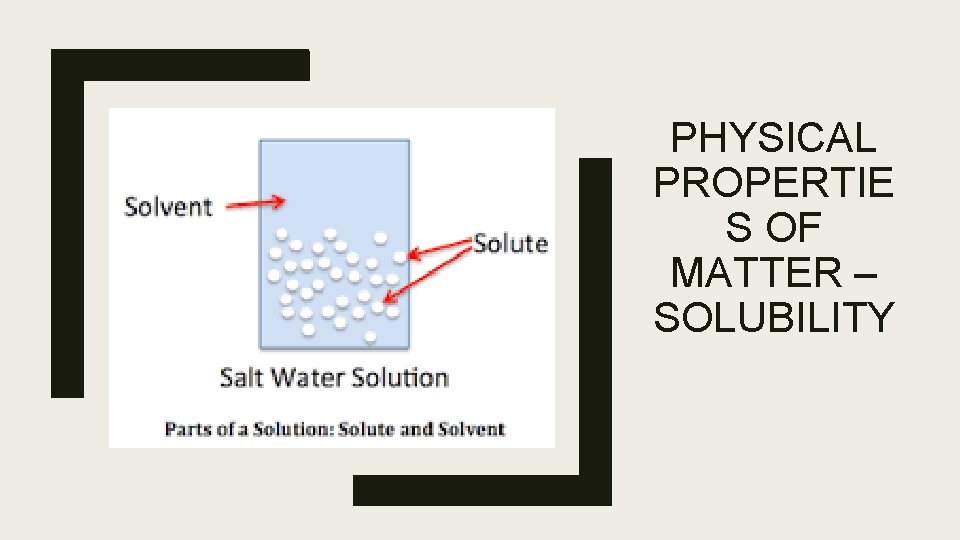
Physical Properties of Matter – Solubility ■ Solubility is the ability of a substance to dissolve into another substance ■ When one thing dissolves into another, a solution has been made ■ There are two parts of a solution: 1. Solute – thing being dissolved 2. Solvent – thing doing the dissolving (usually water) ■ There is more solvent than solute in a solution ■ Something that does not dissolve is called insoluble

PHYSICAL PROPERTIE S OF MATTER – SOLUBILITY

Physical Properties of Matter. Malleability ■ The ability of a substance to be rolled or pounded into a shape https: //www. youtube. com/watch? v=CIBXo. Ya. M 7 Fw

Physical Properties of Matter – Magnetism ■ Magnetism is caused by the motion of electric charges that come from the movement of electrons in atoms ■ Some metals have a magnetic attraction ■ https: //www. youtube. com/watch? v=DEKod. TZOKi. E ■ https: //www. youtube. com/watch? v=MZt. TVs. IOA 9 c

Physical Properties of Matter – Melting Point ■ The melting point of a material is the temperature that it changes from a solid to a liquid ■ Substances with a low melting point will be a liquid at room temperature

Physical Properties of Matter – Boiling Point ■ This is the temperature that a material changes from a liquid to a gas

Chemical Properties ■ This is the ability of a substance to change into a new substance with different properties. ■ Chemical properties can be identified by how the substance changes. ■ Examples of chemical properties are: – flammability (to burn) – reactivity (interacting with another substance) – color change – p. H (how acidic or alkaline something is)

Physical and Chemical Properties of Matter How are they different? ■ Physical properties can be measured without changing a substance ■ When chemical properties are measured they change the substance into something else How are they the same? ■ Physical and chemical properties can be used to identify a substance ■ It does not matter how much of a substance you have, the physical and chemical properties of that substance will stay the same ■ http: //studyjams. scholastic. com/studyjams/science/matter/changes-of-matter. htm

Characteristic Properties ■ These are properties unique to a substance. ■ Take the characteristic properties of copper for example: – Conductor of electricity and heat – Malleable – Lustrous – melting point of 1, 084 degrees Fahrenheit – boiling point of 4, 640 degrees Fahrenheit – density of 8. 96 g/cm^3 – does not react with water – solid at room temperature – not magnetic

Properties of Matter project
 Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 2 type 1
Block nhĩ thất độ 2 type 1 Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Anything that has mass and takes up space is
Anything that has mass and takes up space is Anything that has mass and take up space
Anything that has mass and take up space Matter anything that
Matter anything that Matter is anything that
Matter is anything that All matter has and takes up
All matter has and takes up Matter is anything that
Matter is anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Anything that has mass and takes up space
Anything that has mass and takes up space Ionized matter
Ionized matter Anything that takes up space and has mass
Anything that takes up space and has mass Dmv formula
Dmv formula Matter anything that
Matter anything that Use of density
Use of density Matter is anything that
Matter is anything that