PROPERTIES OF MATTER What is matter Anything that




















- Slides: 24

PROPERTIES OF MATTER

What is matter? ■ Anything that has mass and takes up space ■ Examples: water, air, people, animals, plants, bacteria, rocks, soil, salt

Mass versus weight ■ Mass: – The amount of matter in an object. – Does not change. ■ Weight – How much gravity is pulling down on the mass of an object. – Changes based on gravitational pull.

What is volume? ■ The amount of space an object takes up (occupies) ■ It is measured in different ways: 1) Formula (length x width x height) 2) Water displacement for irregular shapes 3) Graduated cylinder for liquids

Units of Volume ■ Volume is measured as a liter (L) or milliliter (m. L) or cubic centimeter (cm 3) ■ 1 m. L = 1 cm 3

What is density? ■ Density measures the amount of mass in a given volume – Density does not change based on the amount of a material. – Density is a property of that material.

How do you find density? ■ First, you need to know the objects mass (m) in grams and volume (v) in m. L ■ Then you can use this formula to figure out the density: Density = mass / volume The units for density are: grams / m. L (how it is expressed)

Density of Water Density of water = 1 g / m. L or 1 g / cm 3 If an objects density is greater that the density of water, then it will sink. If an objects density is less than water, it will float.

Physical Properties of Matter ■ A characteristic of a substance that can be observed and measured without changing the material. ■ Your senses are used to detect physical properties. ■ What is it called when you use your 5 senses to detect something?

Qualitative vs. Quantitative Observation ■ What is qualitative observation? – Making a descriptive observation about something ■ What is quantitative observation? – Making an observation about something that uses a measurement (numbers)

Physical Properties of Matter ■ The following are common physical properties of matter: – Density – Electrical conductivity – Heat conductivity – Solubility – Malleability – Magnetism – Melting point – Boiling point http: //studyjams. scholastic. com/studyjams/science/matter/properties-of-

Density Mass and Volume ■ Mass is the amount of matter in an object measured in grams – If you change the mass of a material, it will still be that material ■ Volume is the amount of space that a material takes up -If you change the volume of a material, it will still be that same material

Physical Properties of Matter – Electrical Conductivity ■ How well an electrical current moves through something. ■ The ability for an electrical current to move through something does not change based on the amount of that material ■ If a material does not conduct electricity it is known as an insulator https: //www. bing. com/videos/search? q=electrical+conducti vity&&view=detail&mid=7111 D 7 A 21 B 96 C 015 FA 4 E&&FORM=VRDGAR

Physical Properties of Matter – Heat Conductivity ■ How well heat moves through something. ■ The ability for heat to move through something does not change based on the amount of that material https: //www. youtube. com/watch? v=Ry 8 y. Xh. Cxcl. A

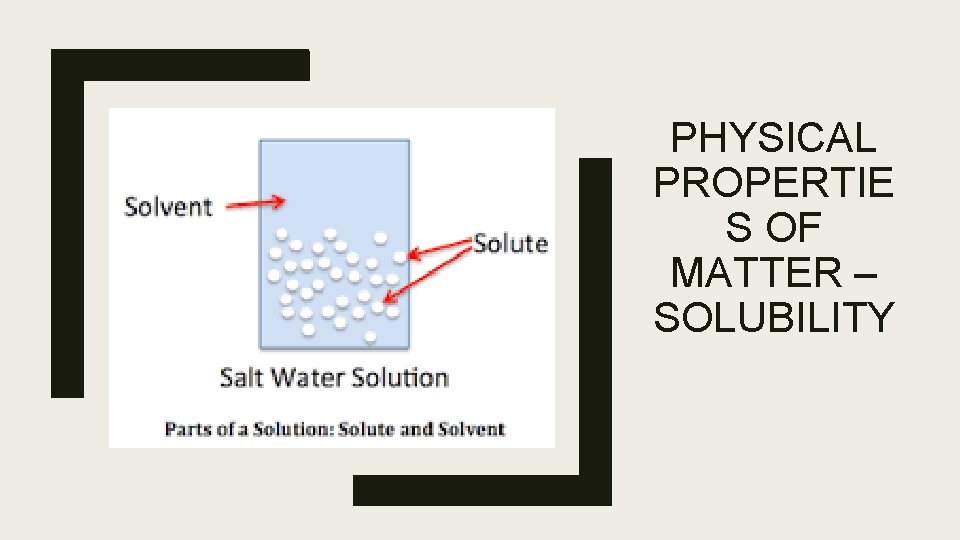
Physical Properties of Matter – Solubility ■ Solubility is the ability of a substance to dissolve into another substance ■ When one thing dissolves into another, a solution has been made ■ There are two parts of a solution: 1. Solute – thing being dissolved 2. Solvent – thing doing the dissolving (usually water) ■ There is more solvent than solute in a solution ■ Something that does not dissolve is called insoluble

PHYSICAL PROPERTIE S OF MATTER – SOLUBILITY

Physical Properties of Matter. Malleability ■ The ability of a substance to be rolled or pounded into a shape https: //www. youtube. com/watch? v=CIBXo. Ya. M 7 Fw

Physical Properties of Matter – Magnetism ■ Magnetism is caused by the motion of electric charges that come from the movement of electrons in atoms ■ Some metals have a magnetic attraction ■ https: //www. youtube. com/watch? v=DEKod. TZOKi. E ■ https: //www. youtube. com/watch? v=MZt. TVs. IOA 9 c

Physical Properties of Matter – Melting Point ■ The melting point of a material is the temperature that it changes from a solid to a liquid ■ Substances with a low melting point will be a liquid at room temperature

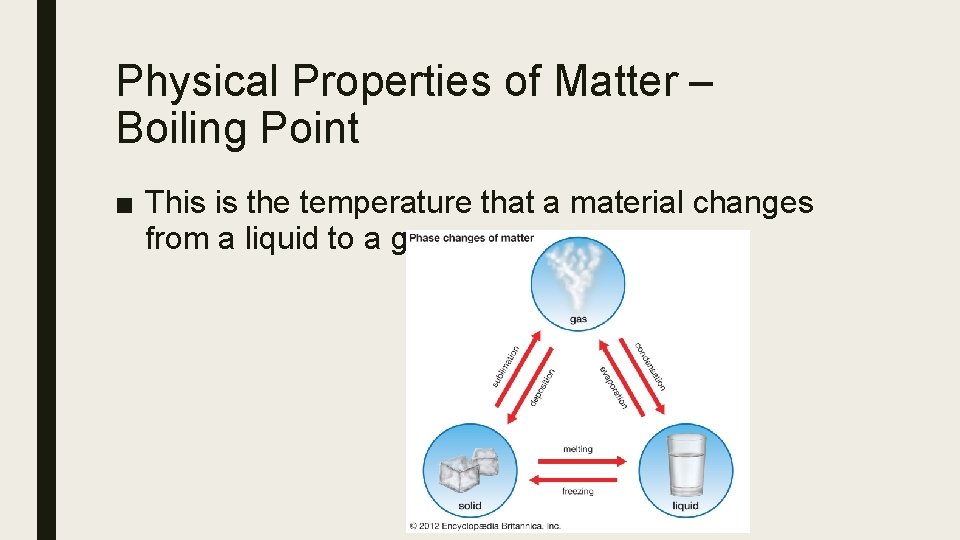
Physical Properties of Matter – Boiling Point ■ This is the temperature that a material changes from a liquid to a gas

Chemical Properties ■ This is the ability of a substance to change into a new substance with different properties. ■ Chemical properties can be identified by how the substance changes. ■ Examples of chemical properties are: – flammability (to burn) – reactivity (interacting with another substance) – color change – p. H (how acidic or alkaline something is)

Physical and Chemical Properties of Matter How are they different? ■ Physical properties can be measured without changing a substance ■ When chemical properties are measured they change the substance into something else How are they the same? ■ Physical and chemical properties can be used to identify a substance ■ It does not matter how much of a substance you have, the physical and chemical properties of that substance will stay the same ■ http: //studyjams. scholastic. com/studyjams/science/matter/changes-of-matter. htm

Characteristic Properties ■ These are properties unique to a substance. ■ Take the characteristic properties of copper for example: – Conductor of electricity and heat – Malleable – Lustrous – melting point of 1, 084 degrees Fahrenheit – boiling point of 4, 640 degrees Fahrenheit – density of 8. 96 g/cm^3 – does not react with water – solid at room temperature – not magnetic

Properties of Matter project