PROPERTIES OF MATTER Volume Mass and Density By

PROPERTIES OF MATTER: Volume, Mass and Density By Caitlyn Reese Unlimited Learning Center, 2015

Please use your mouse to click through each slide. That will allow animations and quizzes to play correctly. Use the back arrow key on your keyboard or the back arrow icon at the bottom left part of each slide to move to previous slides. Move your cursor here to use back arrow

MATTER What is matter? Actually matter is all around you! Even YOU are made of matter! The official definition of matter is any substance that takes up physical space Some examples of things that are not matter include energy, light, sound and heat
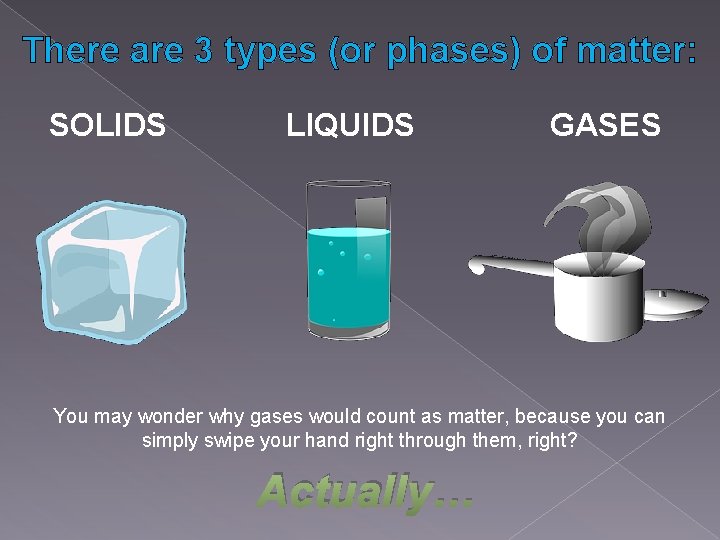
There are 3 types (or phases) of matter: SOLIDS LIQUIDS GASES You may wonder why gases would count as matter, because you can simply swipe your hand right through them, right? Actually…

Gas does take up space! Even though it may not seem like it, gas is made up of millions of tiny atoms just like solids and fluids. (Atoms are the building blocks of all forms of matter, and groups of atoms can join together to form molecules. ) The difference is that the molecules in gases are very spread out! But each molecule is still a physical object that takes up space: You can see this when a gas is compressed (pushed into a smaller space)… Eventually the gas molecules are completely packed together and cannot be compressed any more

VOLUME A defining characteristic of matter is that it has volume What is volume? Of course the first thing you may think of is how loud a sound is, and that is true. But in math and science, volume means something very different! Remember, matter takes up space, and the science and math definition of volume is the amount of THREE-DIMENSIONAL space that a solid, liquid or gas occupies But what do we mean by three-dimensional?
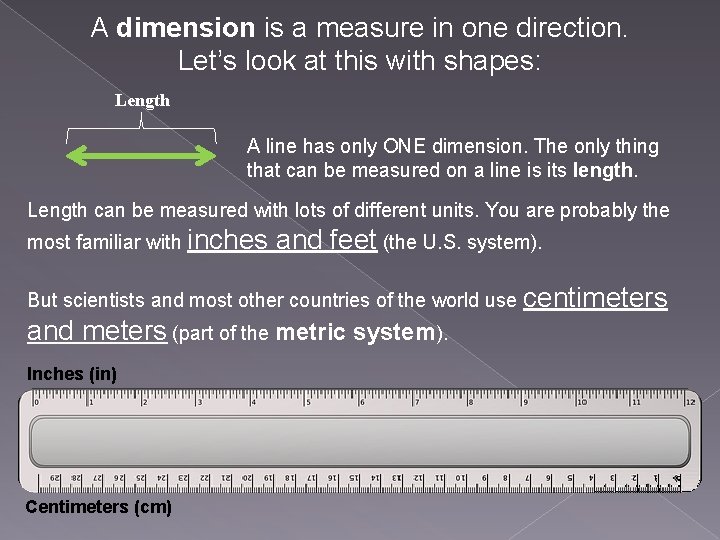
A dimension is a measure in one direction. Let’s look at this with shapes: Length A line has only ONE dimension. The only thing that can be measured on a line is its length. Length can be measured with lots of different units. You are probably the most familiar with inches and feet (the U. S. system). But scientists and most other countries of the world use centimeters and meters (part of the metric system). Inches (in) Centimeters (cm)
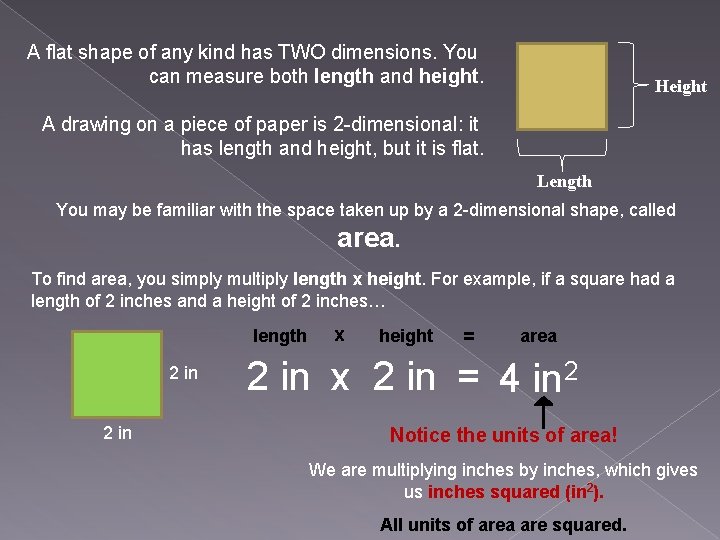
A flat shape of any kind has TWO dimensions. You can measure both length and height. Height A drawing on a piece of paper is 2 -dimensional: it has length and height, but it is flat. Length You may be familiar with the space taken up by a 2 -dimensional shape, called area. To find area, you simply multiply length x height. For example, if a square had a length of 2 inches and a height of 2 inches… length 2 in x height = area 2 in x 2 in = 4 in 2 Notice the units of area! We are multiplying inches by inches, which gives us inches squared (in 2). All units of area are squared.
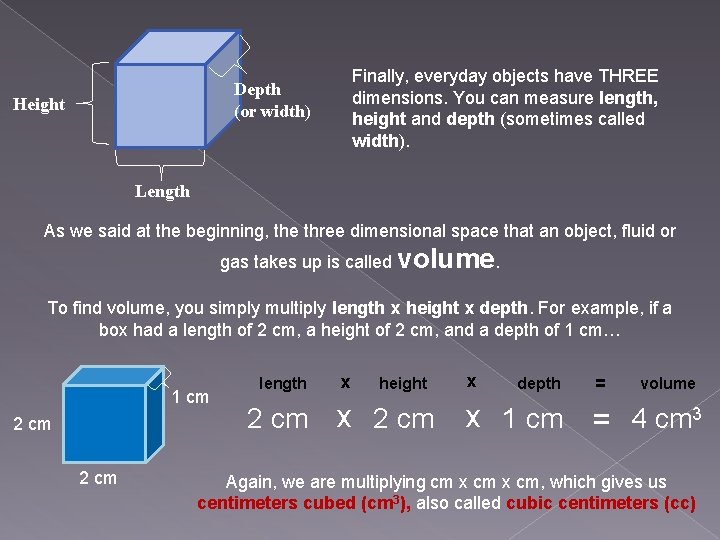
Finally, everyday objects have THREE dimensions. You can measure length, height and depth (sometimes called width). Depth (or width) Height Length As we said at the beginning, the three dimensional space that an object, fluid or gas takes up is called volume. To find volume, you simply multiply length x height x depth. For example, if a box had a length of 2 cm, a height of 2 cm, and a depth of 1 cm… 1 cm 2 cm length 2 cm x height x 2 cm x depth = volume x 1 cm = 4 cm 3 Again, we are multiplying cm x cm, which gives us centimeters cubed (cm 3), also called cubic centimeters (cc)

Click on the link below to play with an interactive tool that uses volume: https: //www. mathsisfun. com/definitions/volume. html After using the “explore” tool, try hitting the “quiz mode” button so you can try to make specific volumes by changing the dimensions!
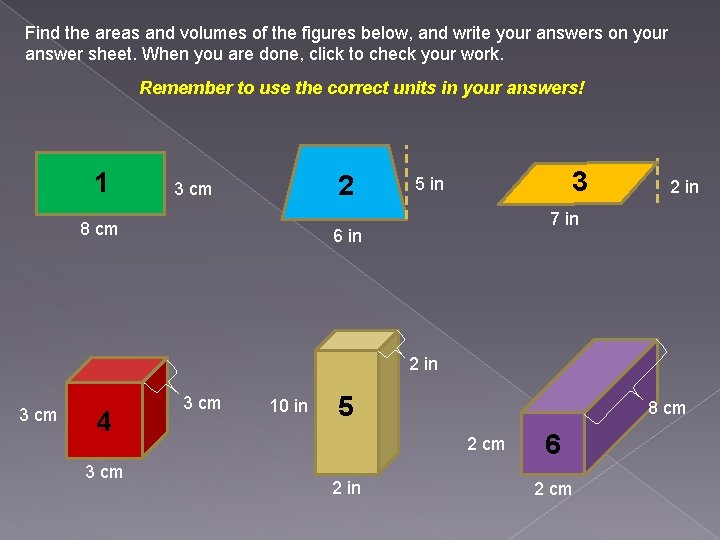
Find the areas and volumes of the figures below, and write your answers on your answer sheet. When you are done, click to check your work. Remember to use the correct units in your answers! 1 2 3 cm 8 cm 3 5 in 2 in 7 in 6 in 2 in 3 cm 4 3 cm 10 in 5 8 cm 2 in 6 2 cm
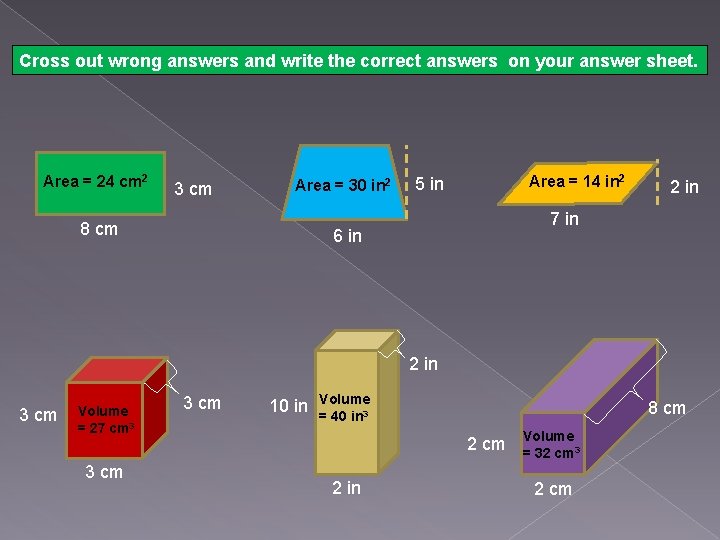
Cross out wrong answers and write the correct answers on your answer sheet. Area = 24 cm 2 3 cm Area = 30 in 2 8 cm Area = 14 in 2 5 in 2 in 7 in 6 in 2 in 3 cm Volume = 27 cm 3 3 cm 10 in Volume = 40 in 3 8 cm 2 in Volume = 32 cm 3 2 cm

UNITS OF VOLUME The units of volume that we’ve covered so far such as cm 3 and in 3, or even m 3 or ft 3 (cubic meters and cubic feet) are usually used for solids. When talking about fluids (which includes both liquids and gases), usually different types of units are used that are specific to volume. Some of these units that you are probably familiar with are: 1 Gallon 12 fl oz Gallons (gal) Fluid ounces (fl oz) Cups (c)
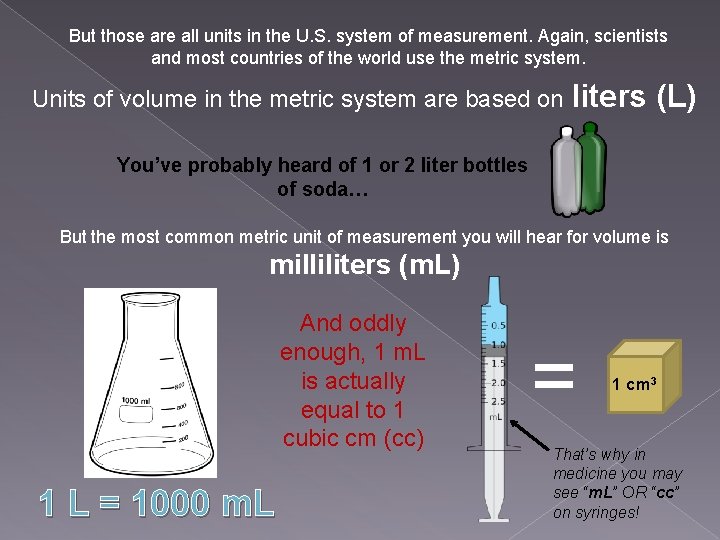
But those are all units in the U. S. system of measurement. Again, scientists and most countries of the world use the metric system. Units of volume in the metric system are based on liters (L) You’ve probably heard of 1 or 2 liter bottles of soda… But the most common metric unit of measurement you will hear for volume is milliliters (m. L) And oddly enough, 1 m. L is actually equal to 1 cubic cm (cc) 1 L = 1000 m. L = 1 cm 3 That’s why in medicine you may see “m. L” OR “cc” on syringes!

CONVERTING m. L and L As you saw on the last slide, metric volumes are based on liters there are 1000 (L), and milliliters (m. L) in 1 liter. The term “milli-” literally So we can also say that 1 m. L is means 1/1000 th of a liter How can we write this in an easier way? We can turn 1/1000 th into a decimal… 1 1000 = 0. 001 Thousandth s place So we see that… 1 m. L = 0. 001 L
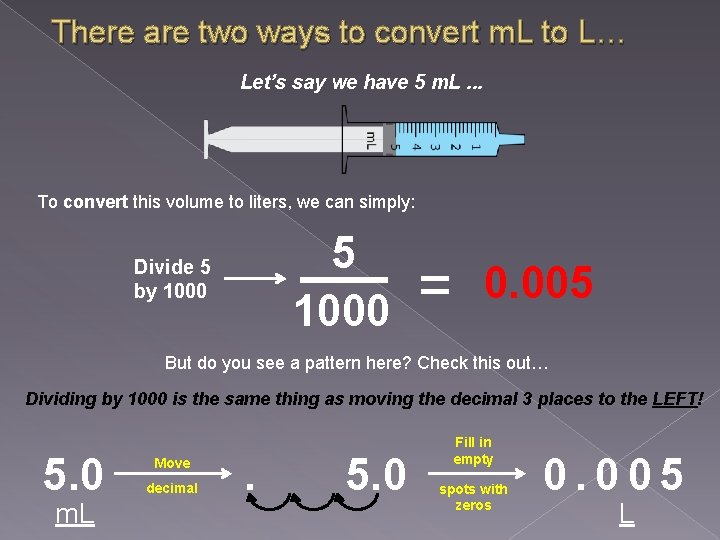
There are two ways to convert m. L to L… Let’s say we have 5 m. L. . . To convert this volume to liters, we can simply: 5 1000 Divide 5 by 1000 = 0. 005 But do you see a pattern here? Check this out… Dividing by 1000 is the same thing as moving the decimal 3 places to the LEFT! 5. 0 m. L Move decimal . 5. 0 Fill in empty spots with zeros 0. 005 L
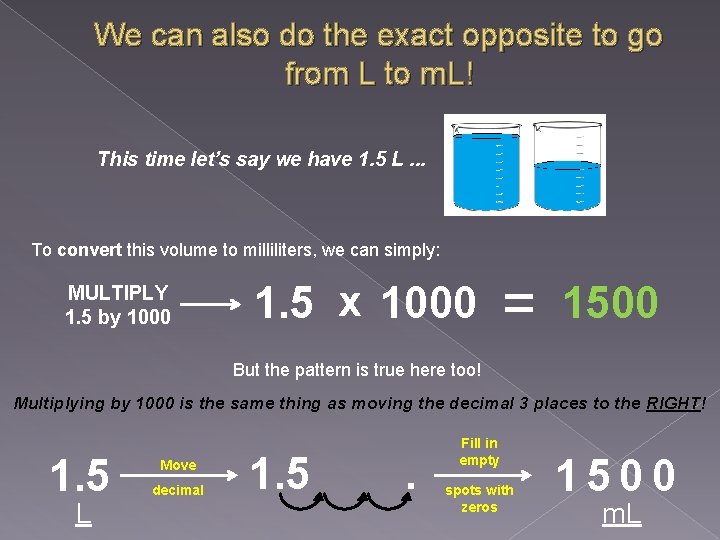
We can also do the exact opposite to go from L to m. L! This time let’s say we have 1. 5 L. . . To convert this volume to milliliters, we can simply: MULTIPLY 1. 5 by 1000 1. 5 x 1000 = 1500 But the pattern is true here too! Multiplying by 1000 is the same thing as moving the decimal 3 places to the RIGHT! 1. 5 L Move decimal 1. 5 . Fill in empty spots with zeros 1500 m. L

YOU CAN USE THIS RULE TO CONVERT BETWEEN ALL METRIC UNITS! For example: Ø Converting between grams and milligrams 1 gram (g) = 1000 milligrams (mg) and 1 mg = 0. 001 g Ø Converting between meters and millimeters 1 meter (m) = 1000 millimeters (mm) and 1 mm = 0. 001 m You just have to remember that: When dividing by 1000 (meaning you’re going from a small to a big unit), you move the decimal point LEFT 3 places and When multiplying by 1000 (meaning you’re going from a big to a small unit) you move the decimal point RIGHT 3 places

Try the following conversions between metric units below, and write your answers on your answer sheet. When you are done, click to check your work. 1) 800 m. L = ? L 2) 1400 m. L = ? L 3) 2. 5 L = ? m. L 4) 0. 06 L = ? m. L 5) 1. 1 g = ? mg 6) 5 mg = ? g 7) 10, 000 mm = ? m

Cross out wrong answers and write the correct answers on your answer sheet. 1) 800 m. L = 0. 8 L 2) 1400 m. L = 1. 4 L 3) 2. 5 L = 2500 m. L 4) 0. 06 L = 60 m. L 5) 1. 1 g = 1100 mg 6) 5 mg = 0. 005 g 7) 10, 000 mm = 10 m

Now that we know what VOLUME is, the units it uses, and how to convert with those units, let’s look at the other defining characteristic of matter…
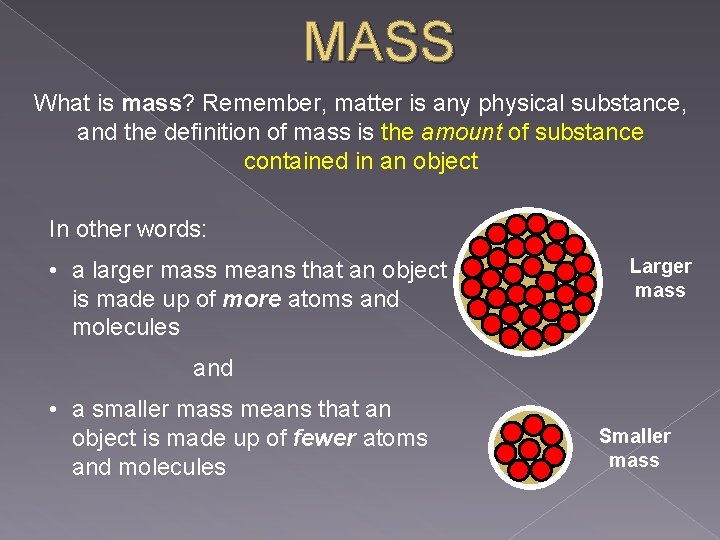
MASS What is mass? Remember, matter is any physical substance, and the definition of mass is the amount of substance contained in an object In other words: • a larger mass means that an object is made up of more atoms and molecules Larger mass and • a smaller mass means that an object is made up of fewer atoms and molecules Smaller mass

UNITS OF MASS Just like length, area and volume, mass can be measured with lots of different units… You are probably the most familiar with pounds U. S. system). and ounces (the But again, scientists and most other countries of the world use kilograms, grams and milligrams (the metric system).

MASS versus WEIGHT Now you might be thinking, “wait a minute, I thought those were the units of weight!” And you’re right! But there is a big difference between mass and weight, and it confuses a lot of people. The definition of weight is actually the force that gravity has on a certain mass This means that an object with the same mass can have a different weight on planets with different amounts of gravity. For example… This is because there is less gravity on the moon, so its force on your mass is less If you weigh 75 kilograms on Earth… You would actually only weigh 12 kilograms on the moon!!!

But, since we are rarely concerned with how much something weighs on another planet, an object’s mass and weight are often used as if they are the same thing. Now that we have that cleared up, let’s talk more about the units of mass… Units of mass in the metric system are based on grams You’ve probably heard of grams or milligrams (mg) when you get medication from your doctor or at the store… And remember, “milli-” actually means 1/1000 th, so… 1 mg = 1/1000 th of a g (g)
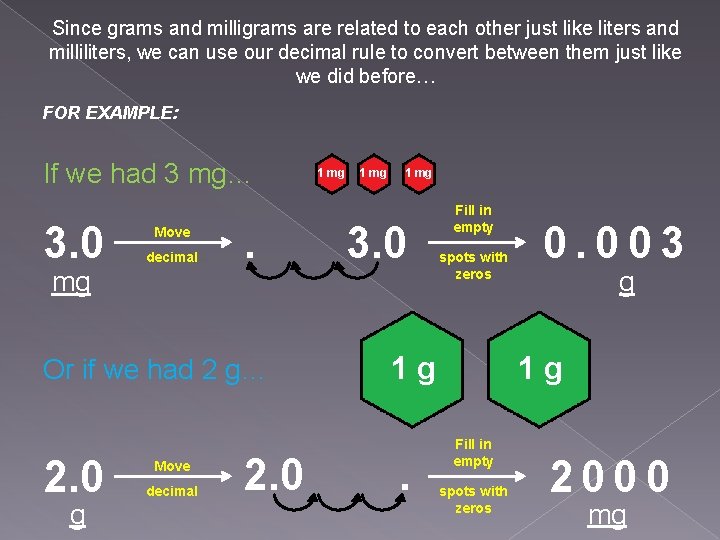
Since grams and milligrams are related to each other just like liters and milliliters, we can use our decimal rule to convert between them just like we did before… FOR EXAMPLE: If we had 3 mg… 3. 0 Move decimal mg . Or if we had 2 g… 2. 0 g Move decimal 2. 0 1 mg 3. 0 Fill in empty spots with zeros 1 g . 0. 003 g 1 g Fill in empty spots with zeros 2000 mg
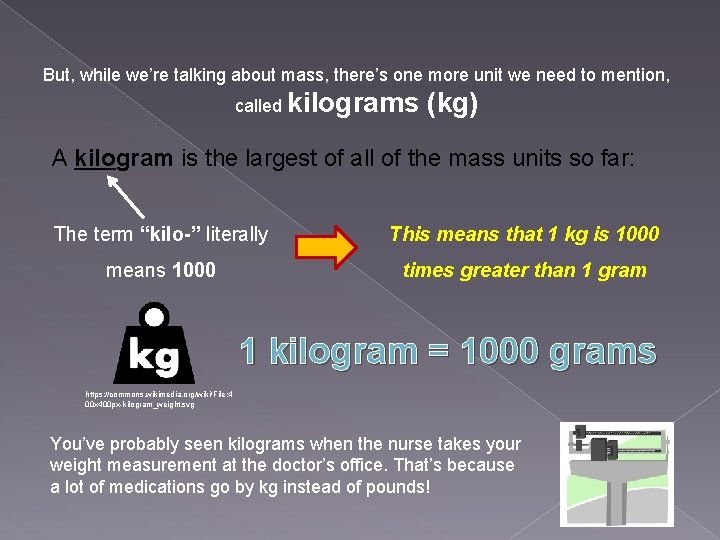
But, while we’re talking about mass, there’s one more unit we need to mention, called kilograms (kg) A kilogram is the largest of all of the mass units so far: The term “kilo-” literally This means that 1 kg is 1000 means 1000 times greater than 1 gram 1 kilogram = 1000 grams https: //commons. wikimedia. org/wiki/File: 4 00 x 400 px-kilogram_weight. svg You’ve probably seen kilograms when the nurse takes your weight measurement at the doctor’s office. That’s because a lot of medications go by kg instead of pounds!

CONVERTING kg, g and mg So now we know that there are 1000 milligrams in a gram, and 1000 grams in 1 kilogram So how many milligrams are in a kilogram? 1000 X 1000 1, 000! = But with our decimal rule its still easy to convert, even between kg and mg… 1500. 0 1 5 0 0. 0 mg . 0015 kg 1. 500 g 1. 5 One more time!

WHEW! That was a lot of info! Try the following conversions between units of mass below, and write your answers on your answer sheet. When you are done, click to check your work. 1) 600 mg = ? g 3) 1. 5 g = ? mg 5) 75 mg = ? g 2) 8 kg = ? g 4) 3500 g = ? kg 6) 0. 45 kg = ? g 7) 2 kg = ? mg
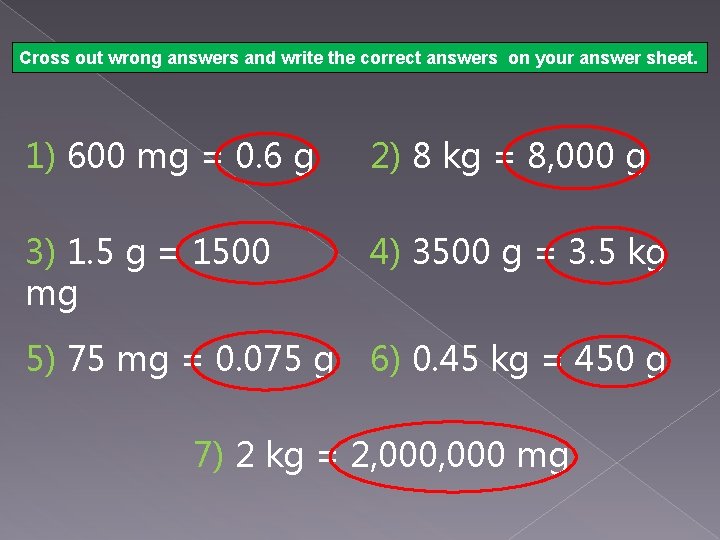
Cross out wrong answers and write the correct answers on your answer sheet. 1) 600 mg = 0. 6 g 2) 8 kg = 8, 000 g 3) 1. 5 g = 1500 mg 4) 3500 g = 3. 5 kg 5) 75 mg = 0. 075 g 6) 0. 45 kg = 450 g 7) 2 kg = 2, 000 mg

Now that we know what VOLUME and MASS are, we can talk about the last characteristic of matter… DENSITY

Density is why… Helium balloons hover in the air… Treasure chests full of gold sink… And giant cruise ships float!

Have you heard of density before? Remember, all matter has both volume and mass… Well, density is the amount of mass a substance has within 1 unit of volume This means that… If you had two boxes that were the same size (volume), but one had more mass (meaning it has more matter) than the other, then… The box with more mass would have the GREATER density

UNITS OF DENSITY Just like everything else we’ve talked about, density can be measured with several different units But no matter what the units are… MASS DENSIT = VOLUME Y The way you say this is “MASS PER VOLUME” And unlike length, volume and mass, people in the U. S. most often use the metric units of density This means we usually don’t have to worry about our own “special units” like pounds, gallons and ounces when talking about density (YAY!)
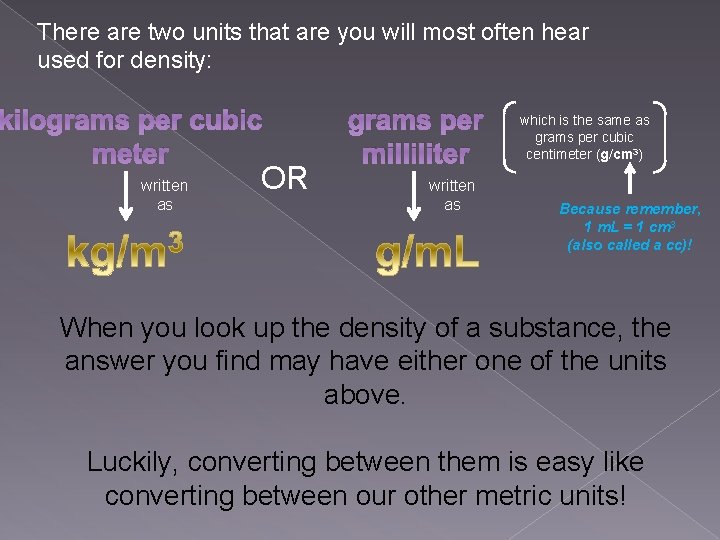
There are two units that are you will most often hear used for density: kilograms per cubic meter written as OR grams per milliliter written as which is the same as grams per cubic centimeter (g/cm 3) Because remember, 1 m. L = 1 cm 3 (also called a cc)! When you look up the density of a substance, the answer you find may have either one of the units above. Luckily, converting between them is easy like converting between our other metric units!
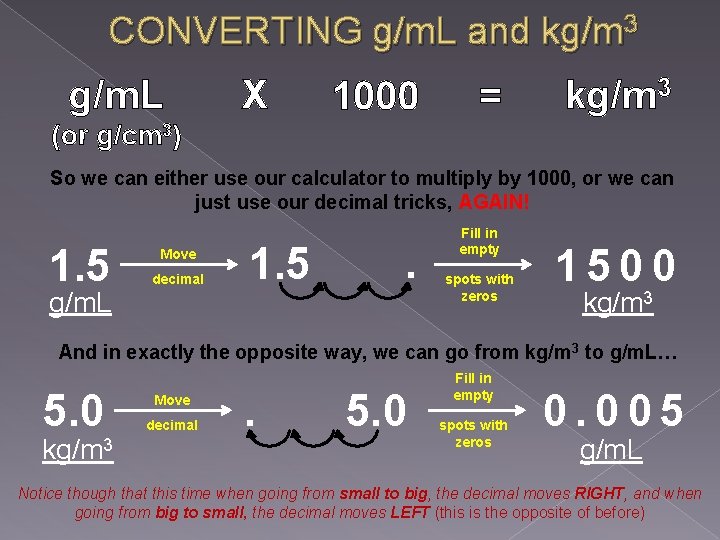
CONVERTING g/m. L and kg/m 3 g/m. L X 1000 = kg/m 3 (or g/cm 3) So we can either use our calculator to multiply by 1000, or we can just use our decimal tricks, AGAIN! 1. 5 g/m. L Move decimal 1. 5 . Fill in empty spots with zeros 1500 kg/m 3 And in exactly the opposite way, we can go from kg/m 3 to g/m. L… 5. 0 kg/m 3 Move decimal . 5. 0 Fill in empty spots with zeros 0. 005 g/m. L Notice though that this time when going from small to big, the decimal moves RIGHT, and when going from big to small, the decimal moves LEFT (this is the opposite of before)

Try the following conversions between units of density below, and write your answers on your answer sheet. When you are done, click to check your work. 1) 2 g/m. L = ? kg/m 3 2) 1600 kg/m 3 = ? g/m. L 3) 450 g/m. L = ? g/cm 3 4) 780 kg/m 3 = ? g/cm 3 5) 30 g/cm 3 = ? kg/m 3 6) 20, 500 kg/m 3 = ? g/m. L

Cross out wrong answers and write the correct answers on your answer sheet. 1) 2 g/m. L = 2000 kg/m 3 2) 1600 kg/m 3 = 1. 6 g/m. L 3) 450 g/m. L = 450 g/cm 3 4) 780 kg/m 3 = 0. 780 g/cm 3 5) 30 g/cm 3 = 30, 000 kg/m 3 6) 20, 500 kg/m 3 = 20. 5 g/m. L

Sink or Float? Water is probably the most important substance when talking about density, because it is a huge part of our lives Water has a density of 1 g/m. L OR 1000 kg/m 3 That’s what it means if you hear someone say “water has a density of 1” – they are talking about 1 g/m. L What’s cool is that density is what determines whether something floats or not! If an object’s density is less than 1, it will FLOAT If an object’s density is greater than 1, it will SINK Click on the link below to play with an awesome simulator showing the density of different materials: https: //phet. colorado. edu/en/simulation/density

What do you think, sink or float? Write your answers on your answer sheet, then click for the answers Ping pong ball Density = 0. 08 g/cm 3 Glass marbles Density = 2. 5 g/cm 3 Ice Density = 0. 92 g/cm 3
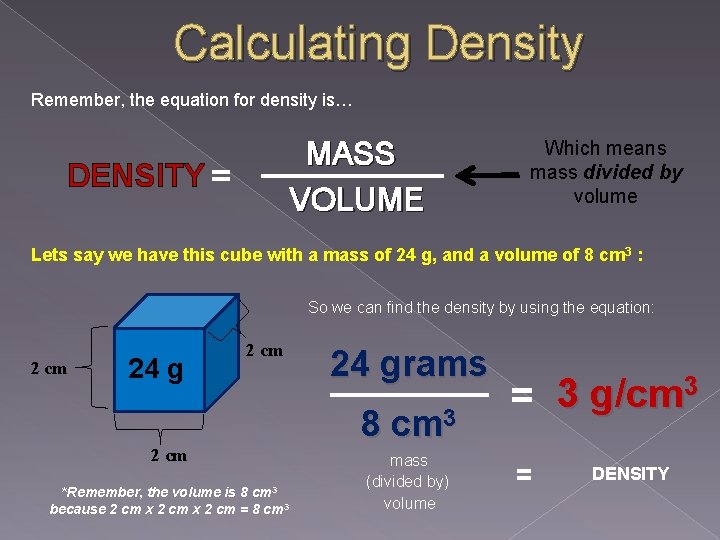
Calculating Density Remember, the equation for density is… MASS VOLUME DENSITY = Which means mass divided by volume Lets say we have this cube with a mass of 24 g, and a volume of 8 cm 3 : So we can find the density by using the equation: 2 cm 24 g 2 cm *Remember, the volume is 8 cm 3 because 2 cm x 2 cm = 8 cm 3 24 grams 8 cm 3 mass (divided by) volume 3 3 g/cm = = DENSITY

Now check out this video to see a summary about density: https: //www. youtube. com/watch? v=Gzd. RGOu. NIFg Note: Mr. Andersen uses the units of kilograms per liter (kg/L) instead of g/m. L, but don’t worry! These are actually exactly the same thing, there is no converting needed. 1 g/m. L = 1 kg/L !!! P. S. Near the end of the video, Mr. Andersen asks you to stop the video, and try some problems. If you want to, great! If not, don’t worry, we’re going to practice our own problems on the next slide.
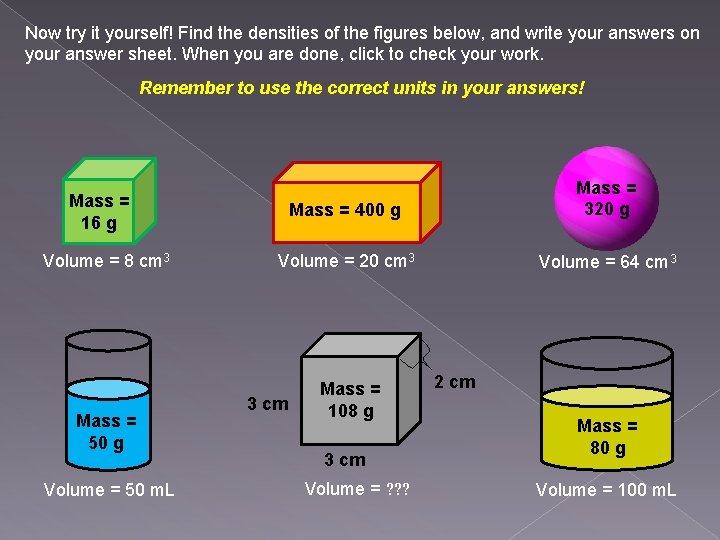
Now try it yourself! Find the densities of the figures below, and write your answers on your answer sheet. When you are done, click to check your work. Remember to use the correct units in your answers! Mass = 400 g Mass = 320 g Volume = 20 cm 3 Volume = 64 cm 3 Mass = 16 g Volume = 8 cm 3 Mass = 50 g Volume = 50 m. L 3 cm Mass = 108 g 3 cm Volume = ? ? ? 2 cm Mass = 80 g Volume = 100 m. L
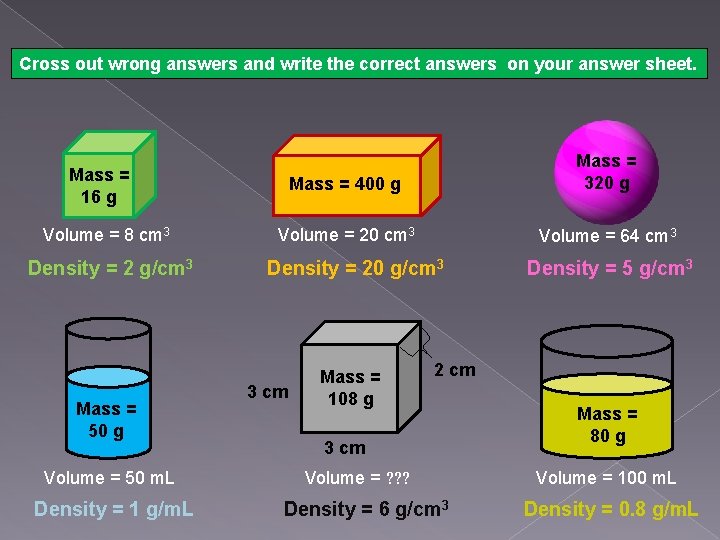
Cross out wrong answers and write the correct answers on your answer sheet. Mass = 400 g Mass = 320 g Volume = 20 cm 3 Volume = 64 cm 3 Mass = 16 g Volume = 8 cm 3 Density = 2 g/cm 3 Mass = 50 g Volume = 50 m. L Density = 1 g/m. L Density = 20 g/cm 3 3 cm Mass = 108 g Density = 5 g/cm 3 2 cm 3 cm Volume = ? ? ? Density = 6 g/cm 3 Mass = 80 g Volume = 100 m. L Density = 0. 8 g/m. L

Review and Practice Turn in your first answer sheet to your coach. Then complete the following worksheets (you should have a print out of them): › › Volume - Rectangular Prism Metric Unit Conversion – Capacity Metric Unit Conversion - Mass Density Calculations Worksheet Once you complete each worksheet, ask your coach for the answer key and correct your work. Don’t worry, you’ll only be graded for completion on this part. Finally, click here to review some flash cards with all of the terms you learned in this lesson (you can also play games with the terms!): https: //quizlet. com/93455601/math-module-12 -properties-ofmatter-flash-cards/

CONGRATULATIONS! You now have a good introduction to mass, volume and density! Ready to take the quiz?
- Slides: 46