PROPERTIES OF MATTER THE PARTICLE MODEL Matter is






- Slides: 11

PROPERTIES OF MATTER

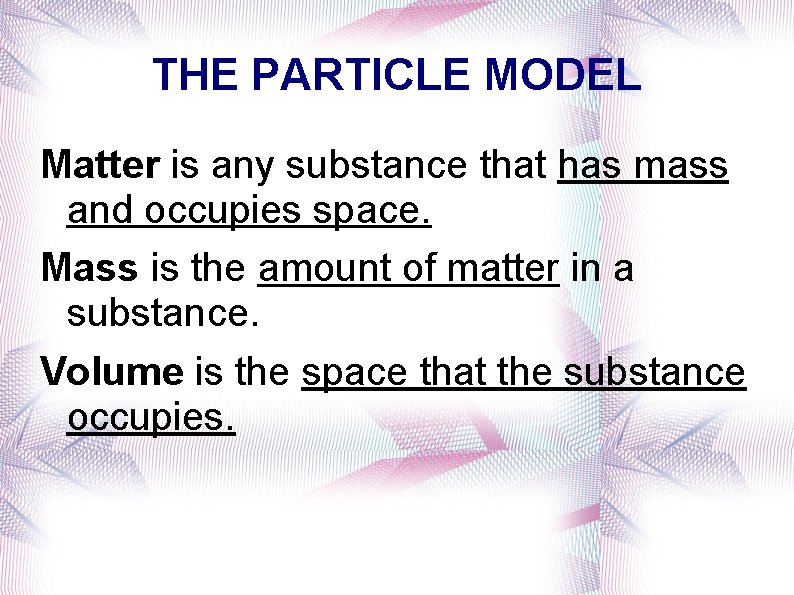
THE PARTICLE MODEL Matter is any substance that has mass and occupies space. Mass is the amount of matter in a substance. Volume is the space that the substance occupies.

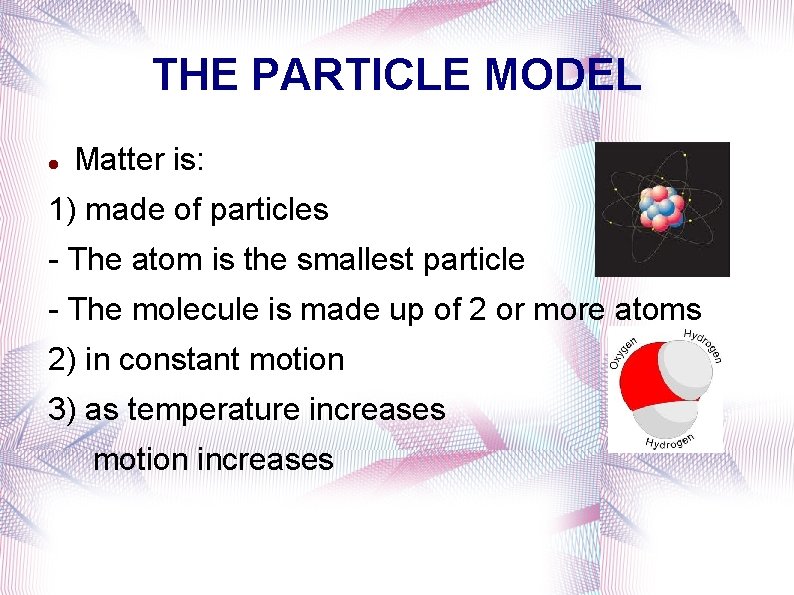
THE PARTICLE MODEL Matter is: 1) made of particles - The atom is the smallest particle - The molecule is made up of 2 or more atoms 2) in constant motion 3) as temperature increases motion increases

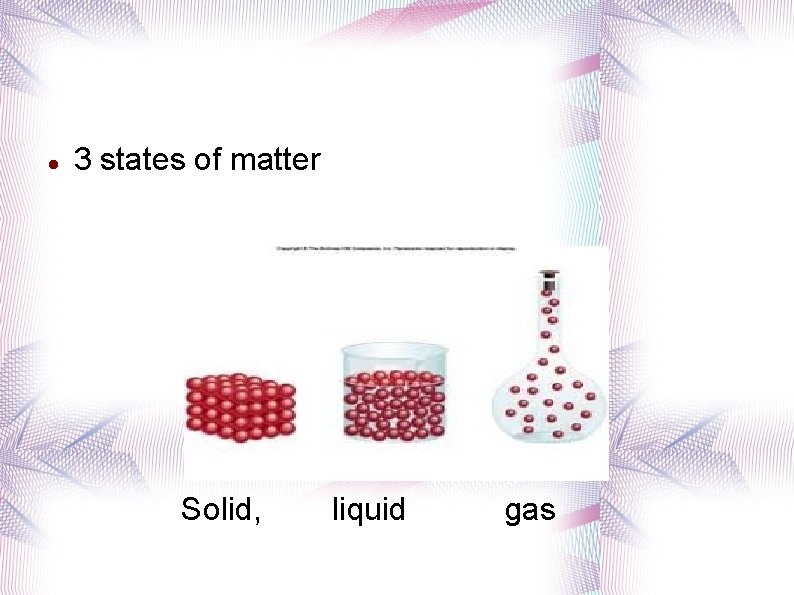
3 states of matter Solid, liquid gas

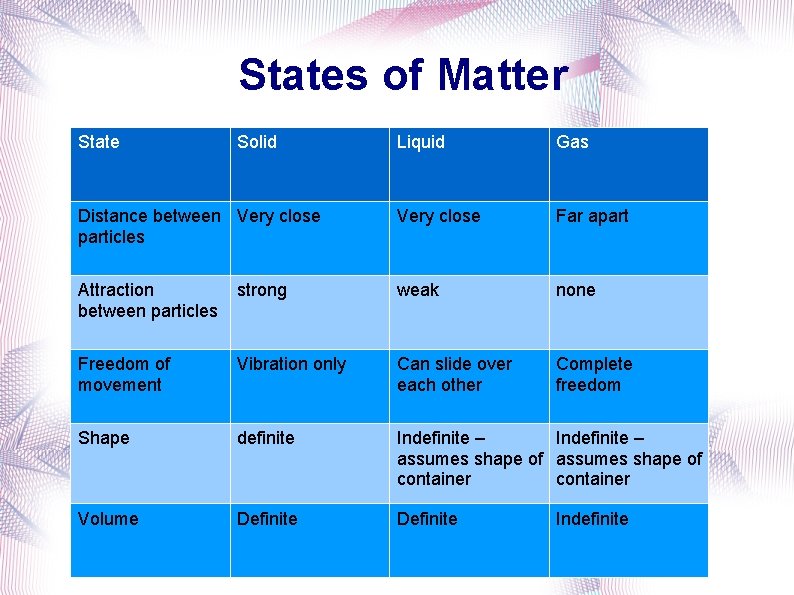
States of Matter State Solid Liquid Gas Distance between Very close particles Very close Far apart Attraction between particles strong weak none Freedom of movement Vibration only Can slide over each other Complete freedom Shape definite Indefinite – assumes shape of container Volume Definite Indefinite

What is a property? A property is a quality that can be observed and that makes it possible to identify an object or a substance.

Physical properties are: -characteristic when they describe a way of being that is specific to a substance or a limited group of substances. Examples – density, melting point, boiling point - non-characteristic when they describe a way of being that is common to a large number of substances. Examples – colour, length, shape, mass, volume

Physical Properties Physical properties can be: -Qualitative like colour, odour, taste, shape, state, etc. -Quantitative (can be measured) like density, melting point, boiling point, solubility in water, etc.

Chemical properties - describe how a substance reacts when it comes in contact with other substances. - describe the new substances formed during the reaction. For example: A chemical property of iron is that it can react with oxygen in a humid environment to form rust (iron oxide). A chemical property of acids is that they will turn blue litmus red on contact. These are also characteristic properties.

Properties of Solids Physical Properties: Examples NCP mass, volume, colour, length, width, height Examples CP: Density, melting point, boiling point, magnetism, malleability, ductility Chemical properties: (Usually destructive to the sample) Example: CP: Flame test,

Magnetism – attractive and repulsive forces between objects Malleability – property of being able to be hammered or shaped without breaking Electrical conductivity – measure of materials ability to conduct electricity Metallic Lustre – a quality of reflecting light