PROPERTIES OF MATTER Physical and Chemical All substances

PROPERTIES OF MATTER Physical and Chemical

¡All substances have properties that we can use to identify them. ¡For example we can identify a person by their face, their voice, height, finger prints, DNA etc. ¡The more of these properties that we can identify, the better we know the person.

¡In a similar way, matter has properties - and there are many of them. ¡There are two basic types of properties that we can associate with matter. ¡These properties are called Physical properties and Chemical properties:

What is a PHYSICAL PROPERTY of matter?
PHYSICAL PROPERTY ¡ Characteristic that can be observed without changing the identity of the substance. ¡ Properties that do not change the chemical nature of matter.

PHYSICAL PROPERTY Examples: l. Color l. Shape l. Texture l. Size l. Taste l. Odor l. Length

TAKE SOME NOTES…
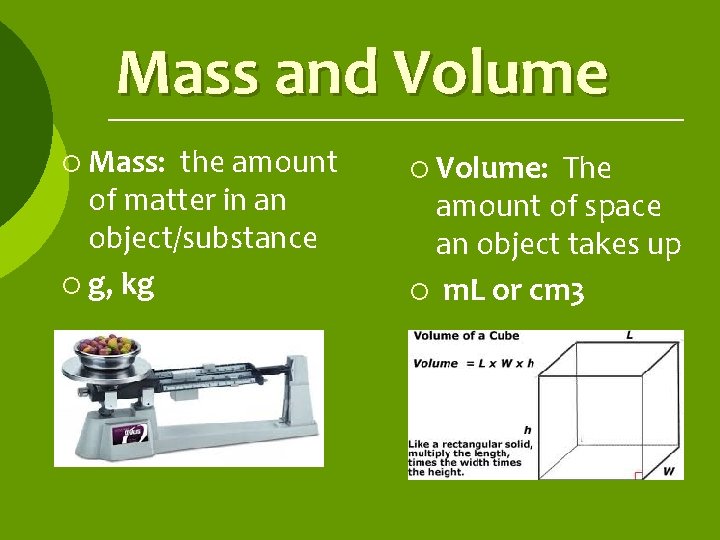
Mass and Volume ¡ Mass: the amount of matter in an object/substance ¡ g, kg ¡ Volume: The amount of space an object takes up ¡ m. L or cm 3

TAKE SOME NOTES…
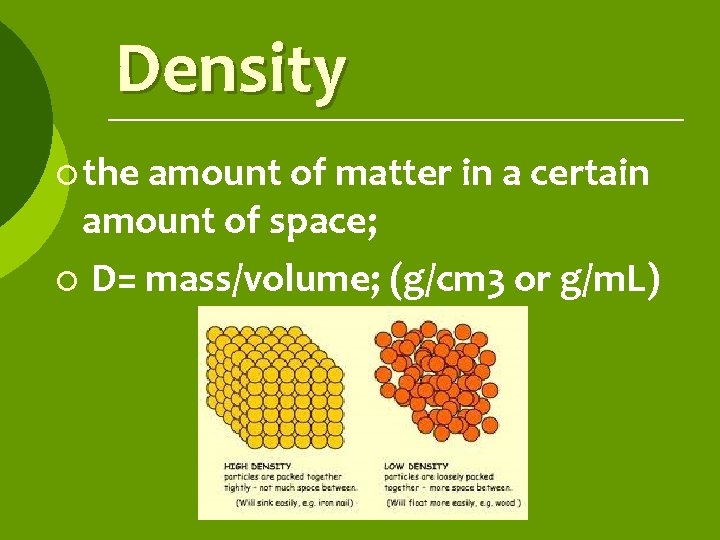
Density ¡ the amount of matter in a certain amount of space; ¡ D= mass/volume; (g/cm 3 or g/m. L)

TAKE SOME NOTES…

ELASTICITY ¡ “stretchy-ness” ¡ the ability to return to original shape

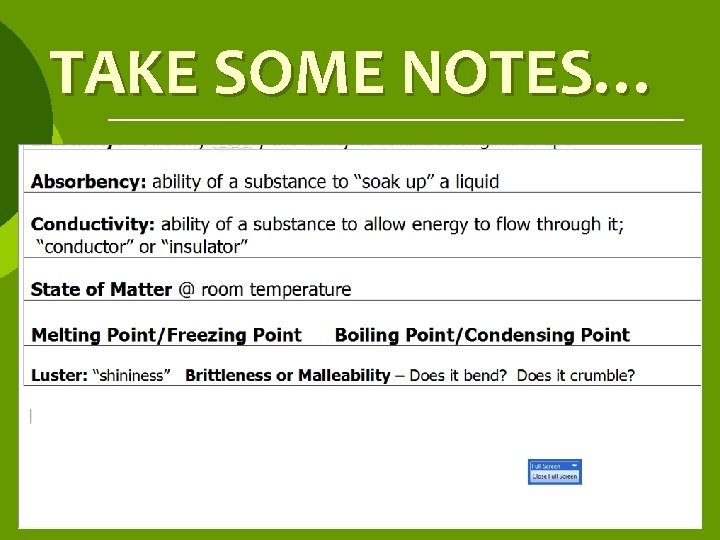
ABSORBENCY ¡Ability of a substance to “soak up” a liquid

TAKE SOME NOTES…

CONDUCTIVITY The ability of a substance to allow energy to flow through it; “conductor” or “insulator”

STATE OF MATTER @ room temperature
SPECIAL TEMPS ¡ ¡ MELTING POINT FREEZING POINT ¡ ¡ BOILING POINT CONDENSING POINT

TAKE SOME NOTES…

LUSTER ¡“shininess” ¡Substances can be described as Shiny or Dull

MALLEABILITY ¡ Malleable means that a substance is bendable ¡ Malleable substances can be shaped and used for tools

BRITTLENESS Beakable, crumbles
TAKE SOME NOTES…

What is a CHEMICAL PROPERTY of matter?

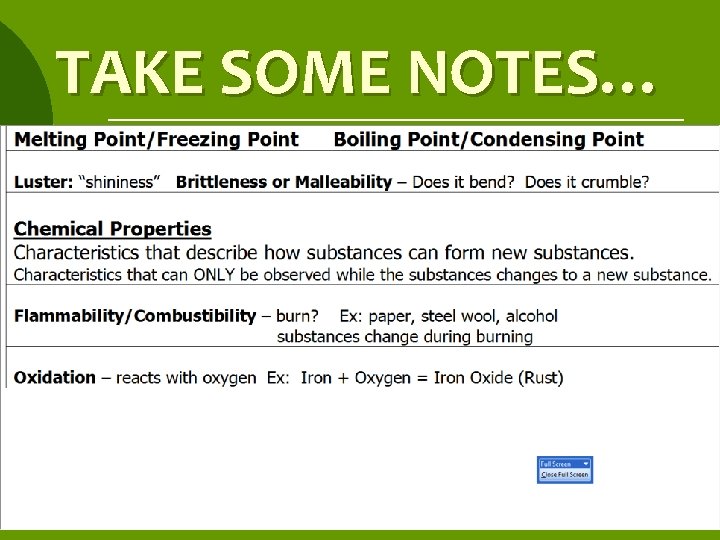
CHEMICAL PROPERTY ¡ Characteristics that describe how substances can form new substances. ¡ Characteristics that can ONLY be observed while the substances changes to a new substance.

COMBUSTIBILITY/ FLAMMABILITY describes how well a substance can burn

OXIDATION: REACTS WITH OXYGEN ¡Iron rusts – forms orange substance. ¡Silver tarnishes – forms dark gray substance. ¡Copper – forms blue/green substance.

TAKE SOME NOTES…

p. H: Acid or Base Acid=Vinegar p. H test is orange/red Ammonia=Base p. H test is blue/purple

REACTIVITY ¡ Reaction with acid? ¡ Baking soda + vinegar ¡ Reaction with H 2 O? ¡ Sodium + water

TAKE SOME NOTES…

What do we use properties of matter for? ¡ To identify substances ¡ To compare substances ¡ To tell one substance from another
- Slides: 32