Properties of Matter Bonding Dr Daniel Reed Office

Properties of Matter Bonding Dr Daniel Reed Office No. : Met&Mat (G 6) 2 B 03 E-Mail: d. reed@bham. ac. uk Properties of matter week 3

Learning outcomes • Bonding • State that noble gasses have a stable electron configuration • Describe different types of bonding • Predict the type of bonding Properties of matter week 3
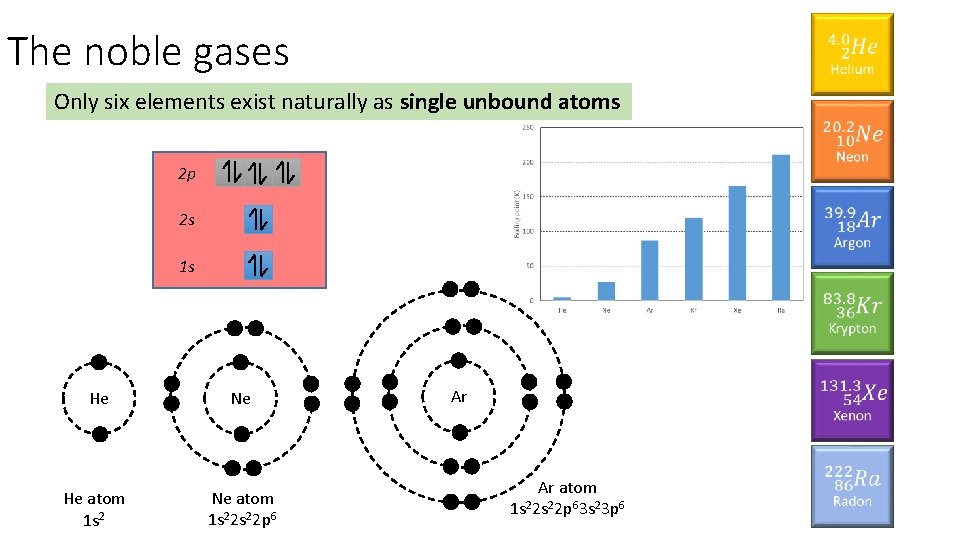
The noble gases Only six elements exist naturally as single unbound atoms 2 p 2 s 1 s He Ne He atom 1 s 2 Ne atom 1 s 22 p 6 Ar Ar atom 1 s 22 p 63 s 23 p 6
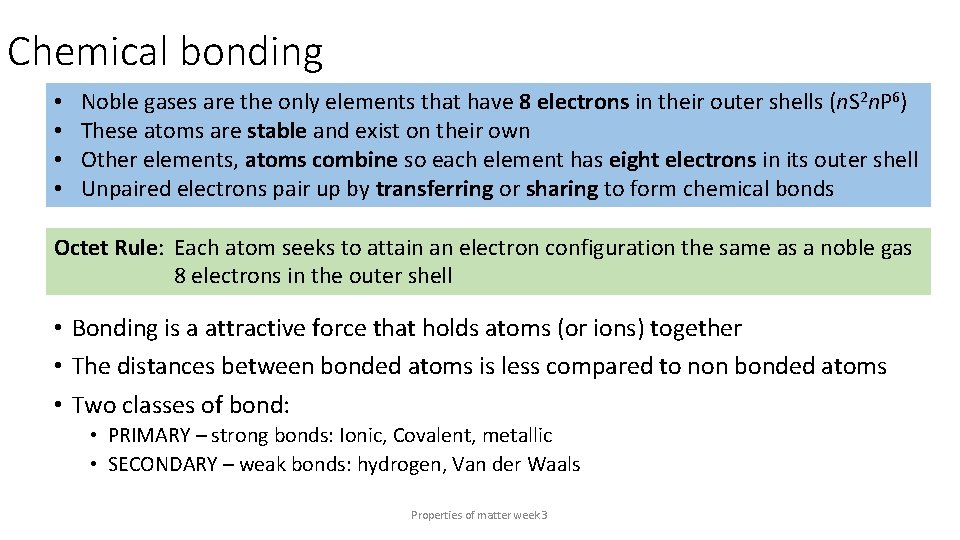
Chemical bonding • • Noble gases are the only elements that have 8 electrons in their outer shells (n. S 2 n. P 6) These atoms are stable and exist on their own Other elements, atoms combine so each element has eight electrons in its outer shell Unpaired electrons pair up by transferring or sharing to form chemical bonds Octet Rule: Each atom seeks to attain an electron configuration the same as a noble gas 8 electrons in the outer shell • Bonding is a attractive force that holds atoms (or ions) together • The distances between bonded atoms is less compared to non bonded atoms • Two classes of bond: • PRIMARY – strong bonds: Ionic, Covalent, metallic • SECONDARY – weak bonds: hydrogen, Van der Waals Properties of matter week 3
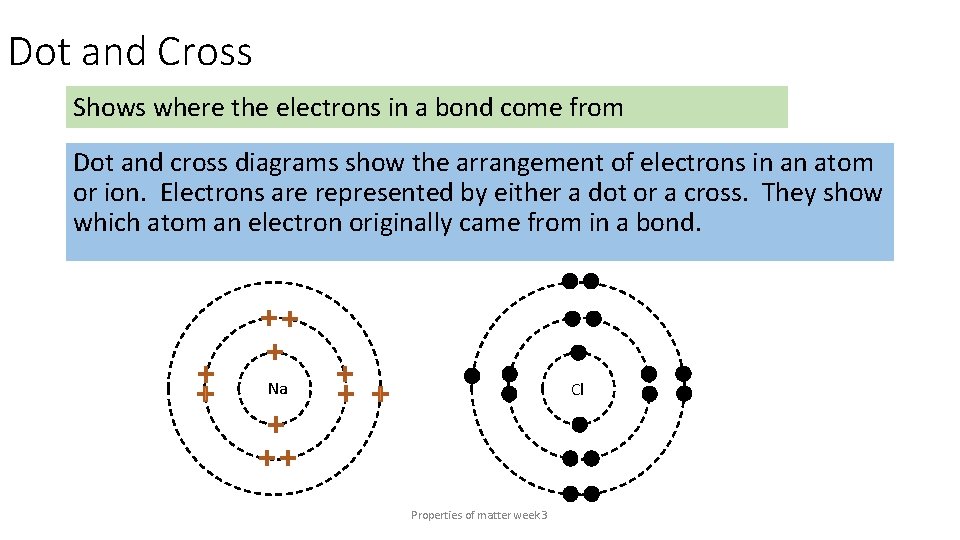
Dot and Cross Shows where the electrons in a bond come from Dot and cross diagrams show the arrangement of electrons in an atom or ion. Electrons are represented by either a dot or a cross. They show which atom an electron originally came from in a bond. Na Cl Properties of matter week 3
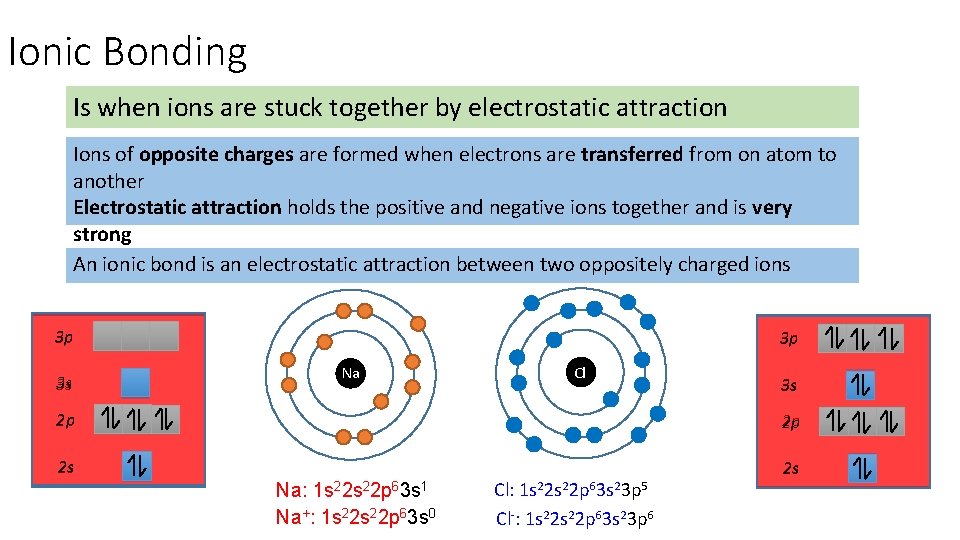
Ionic Bonding Is when ions are stuck together by electrostatic attraction Ions of opposite charges are formed when electrons are transferred from on atom to another Electrostatic attraction holds the positive and negative ions together and is very strong An ionic bond is an electrostatic attraction between two oppositely charged ions 3 p 3 s 3 p Na Cl 3 s 2 p 2 p 2 s 2 s Na: 1 s 22 p 63 s 1 Na+: 1 s 22 p 63 s 0 Cl: 1 s 22 p 63 s 23 p 5 Cl-: 1 s 22 p 63 s 23 p 6
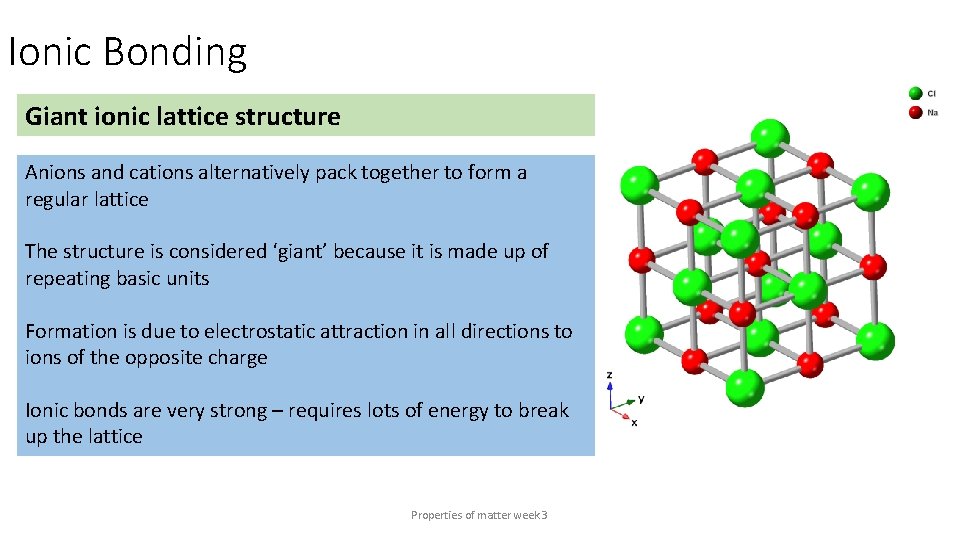
Ionic Bonding Giant ionic lattice structure Anions and cations alternatively pack together to form a regular lattice The structure is considered ‘giant’ because it is made up of repeating basic units Formation is due to electrostatic attraction in all directions to ions of the opposite charge Ionic bonds are very strong – requires lots of energy to break up the lattice Properties of matter week 3
Ionic Bonding Behaviour of ionic compounds 1. Ionic compounds conduct electricity when they are molten or dissolved – but not when they are solid • Ions within a liquid are mobile (and carry charge) • in the solid state ions are normally fixed in position by strong ionic bonds 2. Ionic compounds have high melting and boiling points • The giant ionic lattices are held together by strong electrostatic forces • Requires energy to overcome these forces and melt these materials Properties of matter week 3
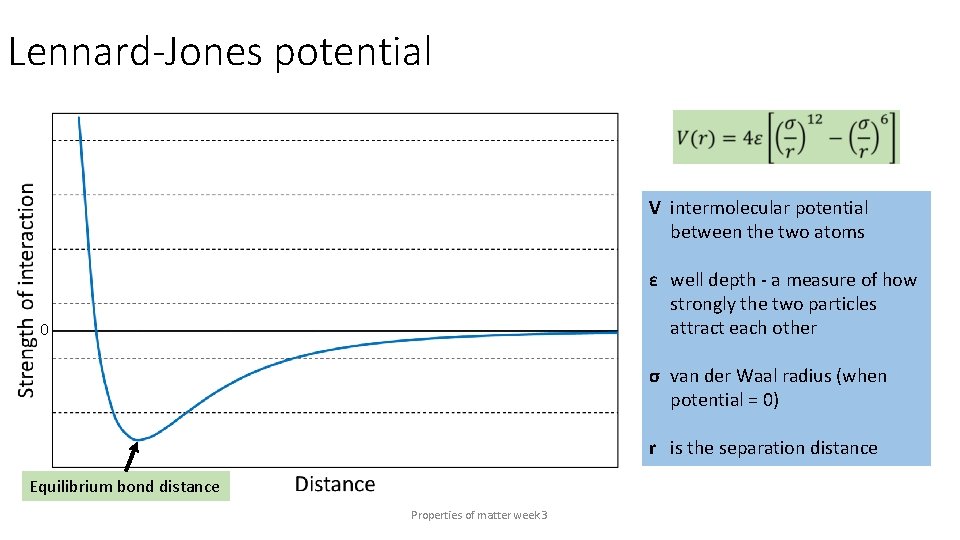
Lennard-Jones potential V intermolecular potential between the two atoms ε well depth - a measure of how strongly the two particles attract each other 0 σ van der Waal radius (when potential = 0) r is the separation distance Equilibrium bond distance Properties of matter week 3
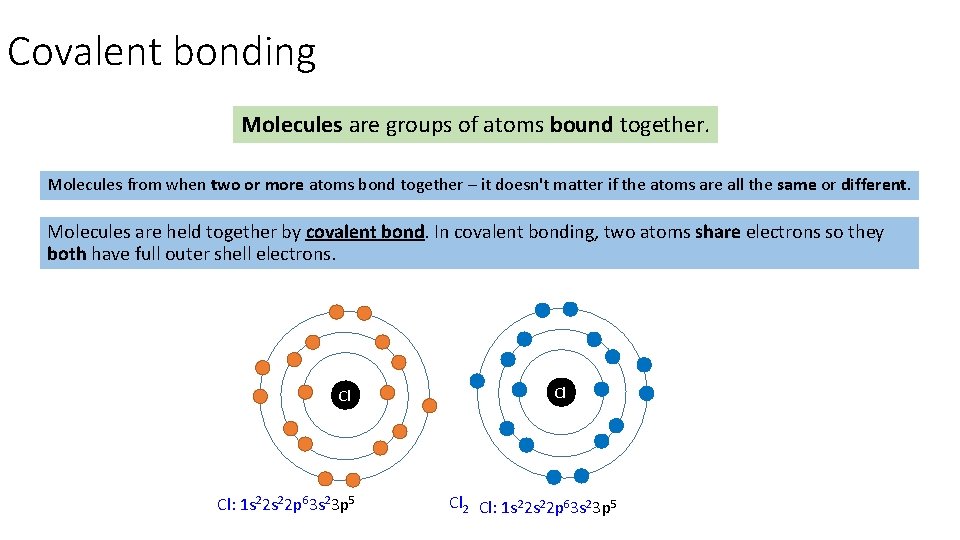
Covalent bonding Molecules are groups of atoms bound together. Molecules from when two or more atoms bond together – it doesn't matter if the atoms are all the same or different. Molecules are held together by covalent bond. In covalent bonding, two atoms share electrons so they both have full outer shell electrons. Cl Cl: 1 s 22 p 63 s 23 p 5 Cl Cl 2 Cl: 1 s 22 p 63 s 23 p 5
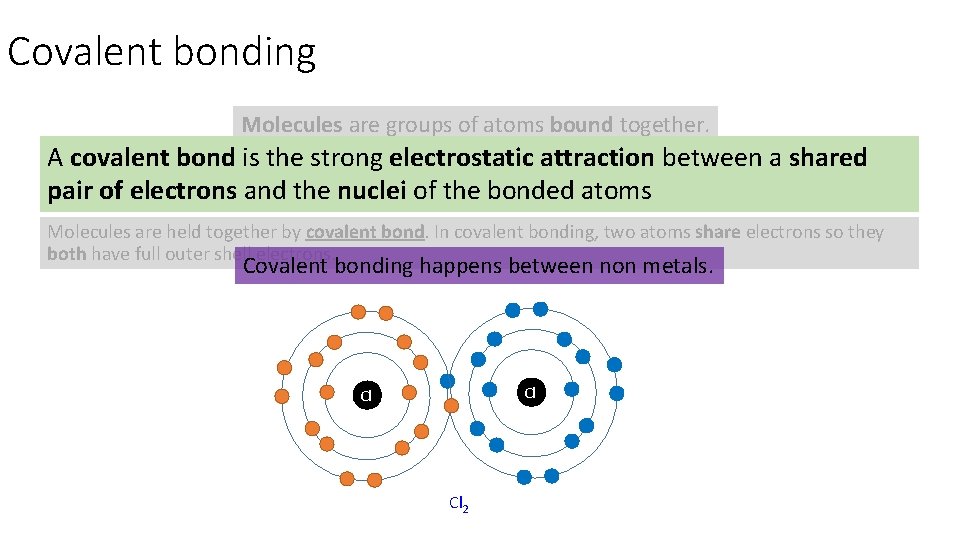
Covalent bonding Molecules are groups of atoms bound together. A covalent bond is the strong electrostatic attraction between a shared Molecules from when two or more atoms bond together – it doesn't matter if the atoms are all the same or different. pair of electrons and the nuclei of the bonded atoms Molecules are held together by covalent bond. In covalent bonding, two atoms share electrons so they both have full outer shell electrons. Covalent bonding happens between non metals. Cl Cl Cl 2
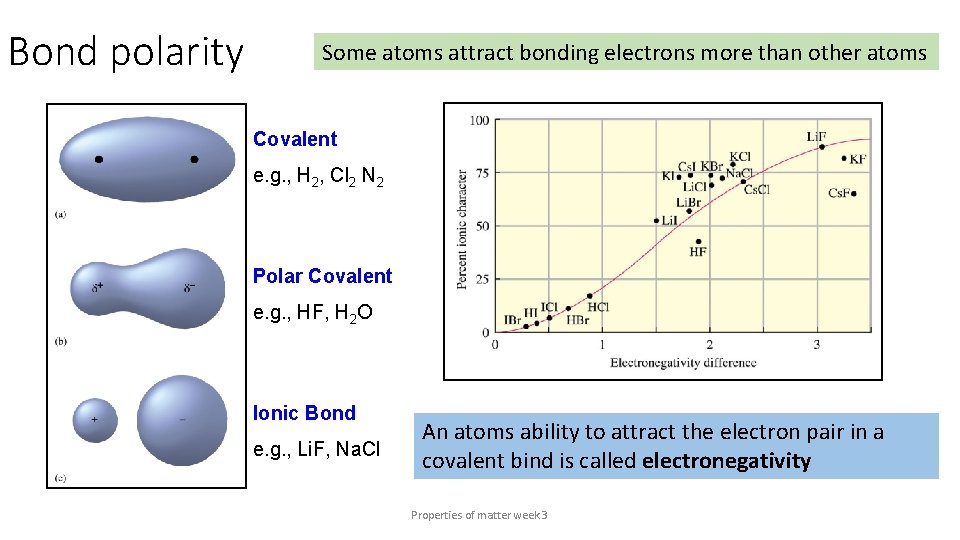
Bond polarity Some atoms attract bonding electrons more than other atoms Covalent e. g. , H 2, Cl 2 N 2 Polar Covalent e. g. , HF, H 2 O Ionic Bond e. g. , Li. F, Na. Cl An atoms ability to attract the electron pair in a covalent bind is called electronegativity Properties of matter week 3
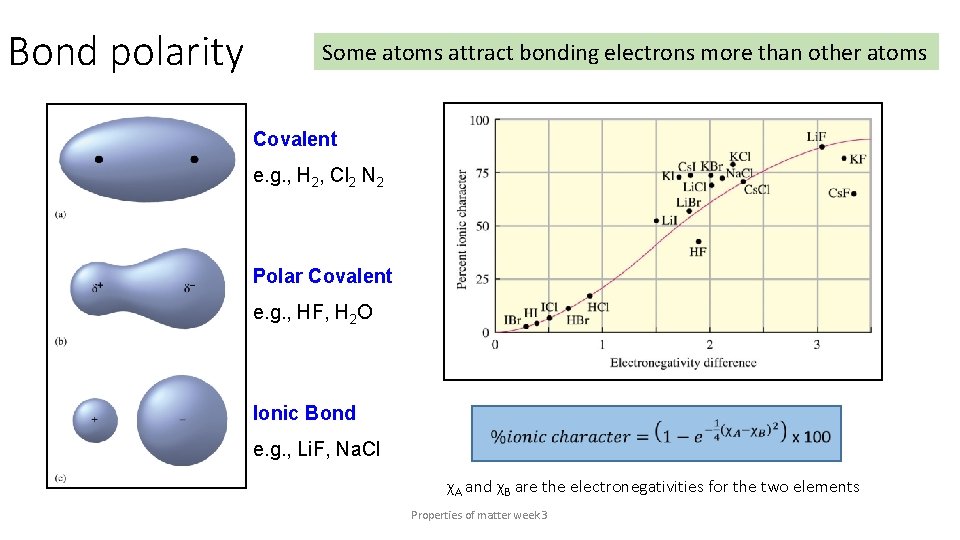
Bond polarity Some atoms attract bonding electrons more than other atoms Covalent e. g. , H 2, Cl 2 N 2 Polar Covalent e. g. , HF, H 2 O Ionic Bond e. g. , Li. F, Na. Cl χA and χB are the electronegativities for the two elements Properties of matter week 3
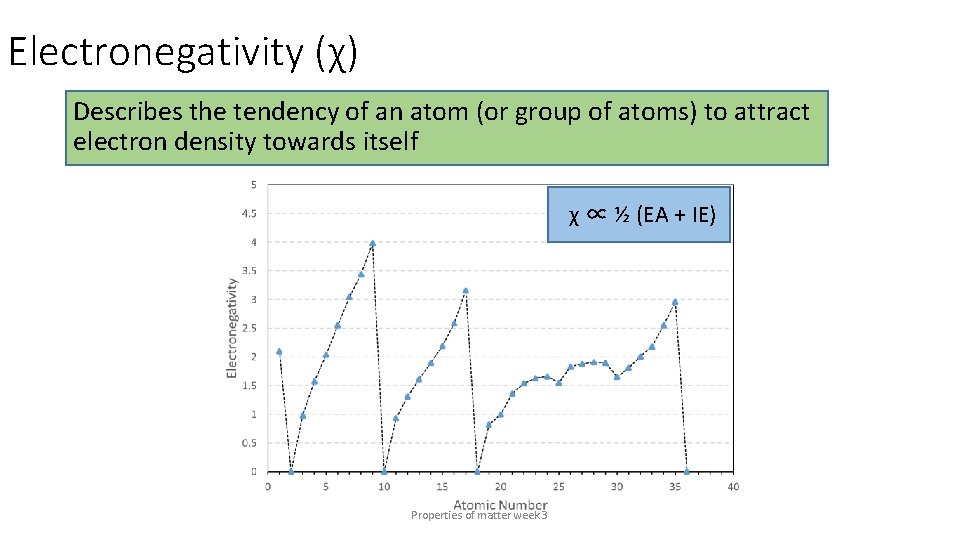
Electronegativity (χ) Describes the tendency of an atom (or group of atoms) to attract electron density towards itself χ ∝ ½ (EA + IE) Properties of matter week 3
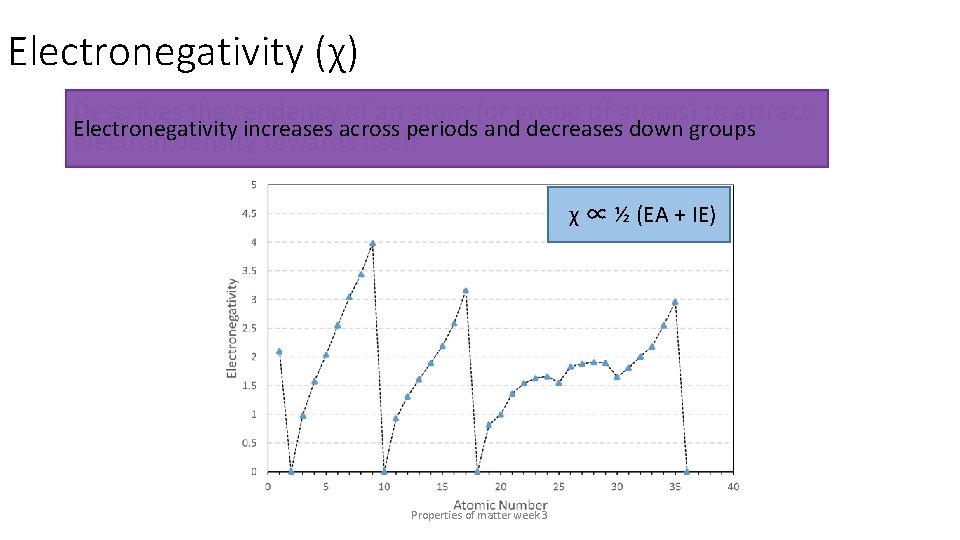
Electronegativity (χ) Describes the tendency of an atom (or group of atoms) to attract Electronegativity increases across periods and decreases down groups electron density towards itself χ ∝ ½ (EA + IE) Properties of matter week 3
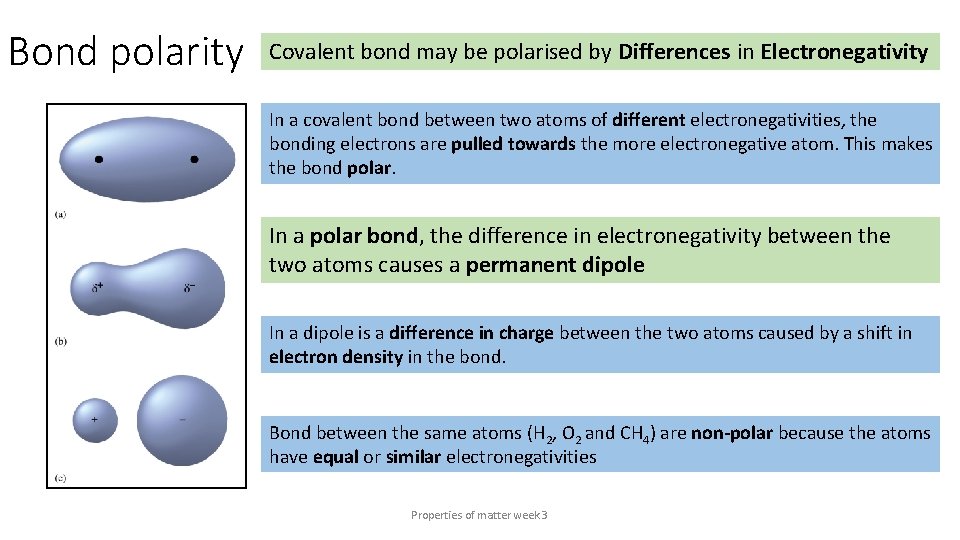
Bond polarity Covalent bond may be polarised by Differences in Electronegativity In a covalent bond between two atoms of different electronegativities, the bonding electrons are pulled towards the more electronegative atom. This makes the bond polar. In a polar bond, the difference in electronegativity between the two atoms causes a permanent dipole In a dipole is a difference in charge between the two atoms caused by a shift in electron density in the bond. Bond between the same atoms (H 2, O 2 and CH 4) are non-polar because the atoms have equal or similar electronegativities Properties of matter week 3
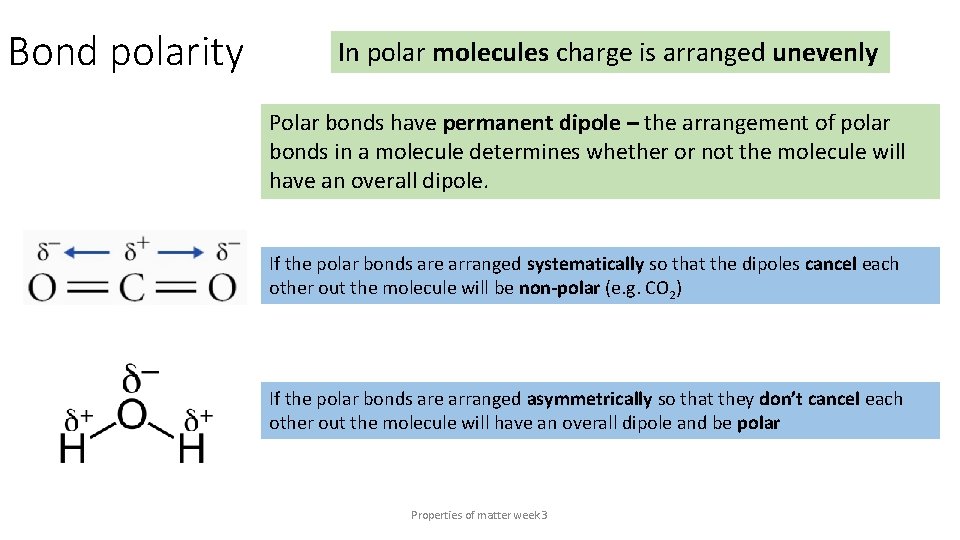
Bond polarity In polar molecules charge is arranged unevenly Polar bonds have permanent dipole – the arrangement of polar bonds in a molecule determines whether or not the molecule will have an overall dipole. If the polar bonds are arranged systematically so that the dipoles cancel each other out the molecule will be non-polar (e. g. CO 2) If the polar bonds are arranged asymmetrically so that they don’t cancel each other out the molecule will have an overall dipole and be polar Properties of matter week 3
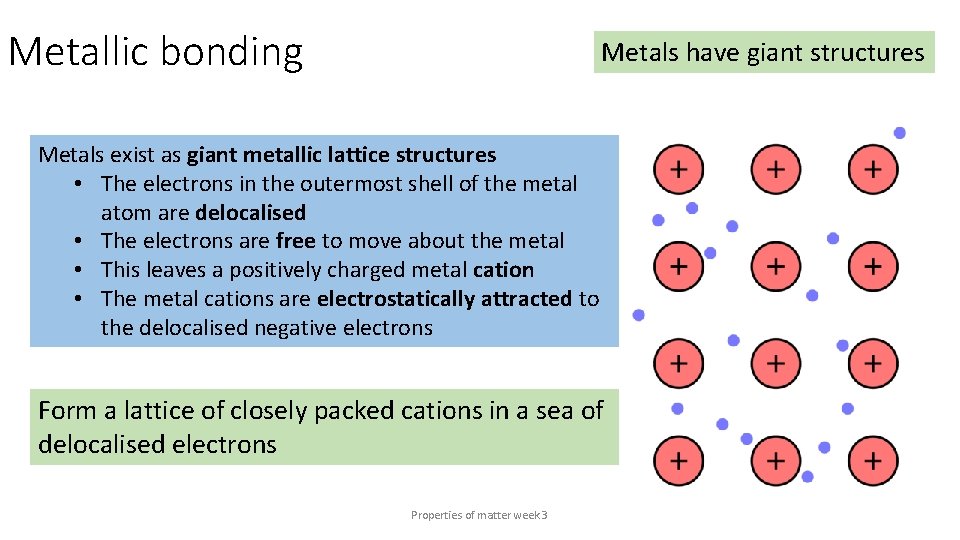
Metallic bonding Metals have giant structures Metals exist as giant metallic lattice structures • The electrons in the outermost shell of the metal atom are delocalised • The electrons are free to move about the metal • This leaves a positively charged metal cation • The metal cations are electrostatically attracted to the delocalised negative electrons Form a lattice of closely packed cations in a sea of delocalised electrons Properties of matter week 3
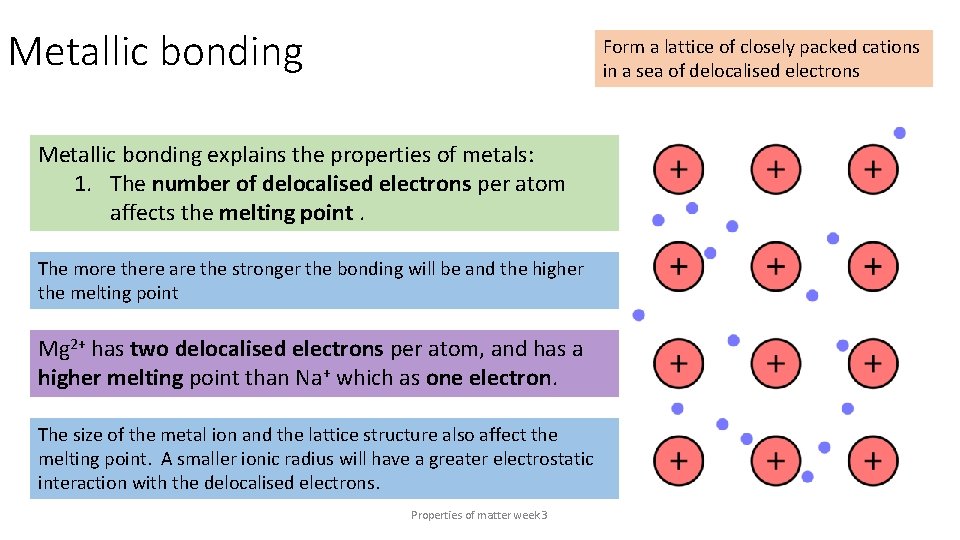
Metallic bonding Form a lattice of closely packed cations in a sea of delocalised electrons Metallic bonding explains the properties of metals: 1. The number of delocalised electrons per atom affects the melting point. The more there are the stronger the bonding will be and the higher the melting point Mg 2+ has two delocalised electrons per atom, and has a higher melting point than Na+ which as one electron. The size of the metal ion and the lattice structure also affect the melting point. A smaller ionic radius will have a greater electrostatic interaction with the delocalised electrons. Properties of matter week 3
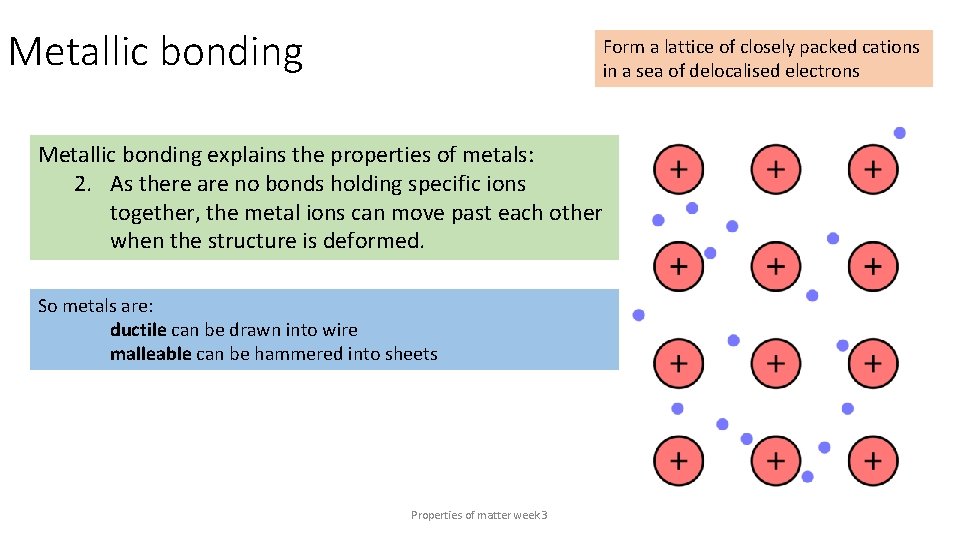
Metallic bonding Form a lattice of closely packed cations in a sea of delocalised electrons Metallic bonding explains the properties of metals: 2. As there are no bonds holding specific ions together, the metal ions can move past each other when the structure is deformed. So metals are: ductile can be drawn into wire malleable can be hammered into sheets Properties of matter week 3
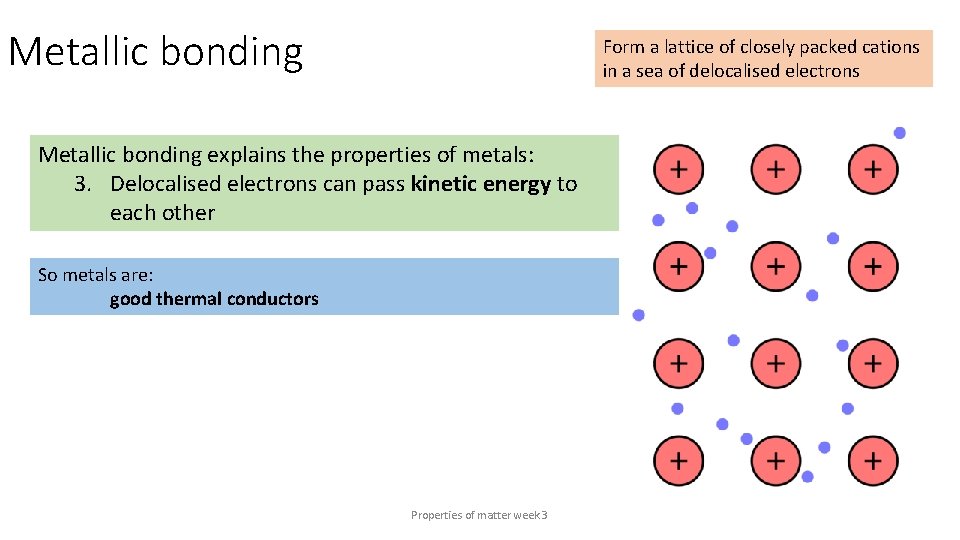
Metallic bonding Form a lattice of closely packed cations in a sea of delocalised electrons Metallic bonding explains the properties of metals: 3. Delocalised electrons can pass kinetic energy to each other So metals are: good thermal conductors Properties of matter week 3
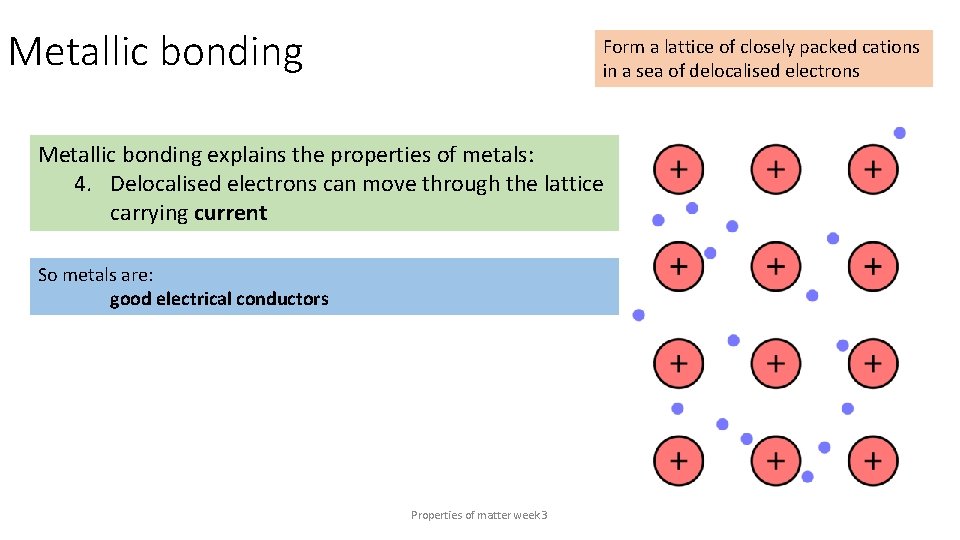
Metallic bonding Form a lattice of closely packed cations in a sea of delocalised electrons Metallic bonding explains the properties of metals: 4. Delocalised electrons can move through the lattice carrying current So metals are: good electrical conductors Properties of matter week 3
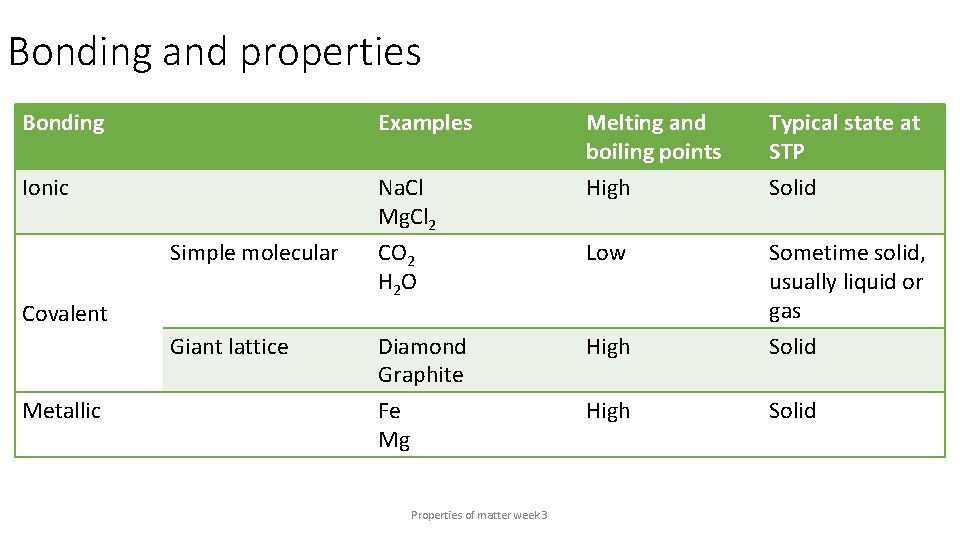
Bonding and properties Bonding Examples Ionic Na. Cl Mg. Cl 2 Typical state at STP Solid Simple molecular CO 2 H 2 O Low Giant lattice Diamond Graphite High Sometime solid, usually liquid or gas Solid Fe Mg High Solid Covalent Metallic Melting and boiling points High Properties of matter week 3

Secondary bonding Intermolecular forces are very weak 1. van der Waal’s forces a) Induced dipole-dipole forces b) Permanent dipole-dipole interactions 2. Hydrogen bonding Properties of matter week 3
Induced Dipole-Dipole forces Found between all atoms and molecules Induced dipole-dipole forces cause all atoms and molecules to be attracted to each other • Electron in charge clouds are always moving (very quickly) • At any given moment the electrons in a molecule can potentially be on one side than the other – this induces a temporary dipole • This dipole can induce another temporary dipole – two dipoles are then attracted to each other – and so on in a domino effect Induced dipole-dipole forces are responsible for holding iodine molecules (I 2) in a lattice • Strong I-I covalent bonds form molecules • Molecules are then held together in a molecular lattice arrangement by weak induced dipole-dipole attractions Properties of matter week 3
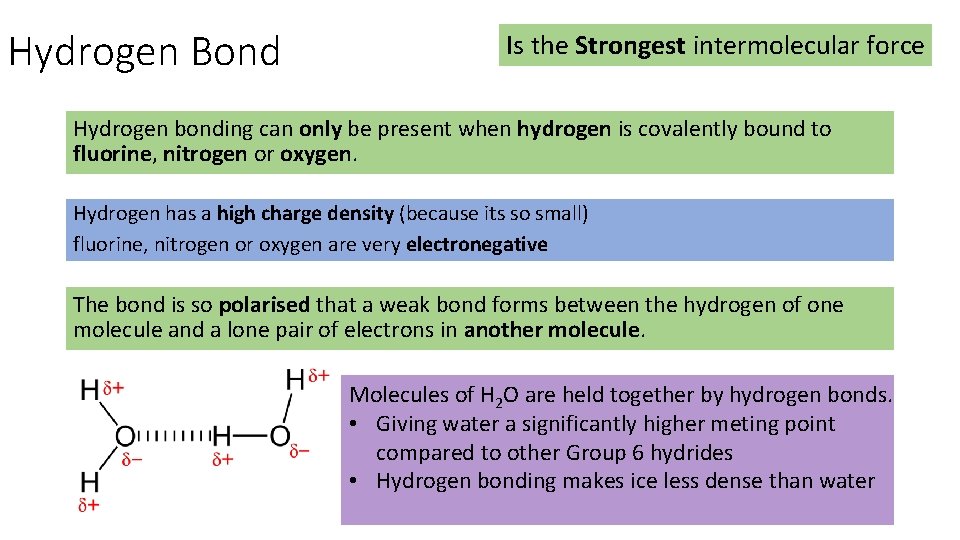
Hydrogen Bond Is the Strongest intermolecular force Hydrogen bonding can only be present when hydrogen is covalently bound to fluorine, nitrogen or oxygen. Hydrogen has a high charge density (because its so small) fluorine, nitrogen or oxygen are very electronegative The bond is so polarised that a weak bond forms between the hydrogen of one molecule and a lone pair of electrons in another molecule. Molecules of H 2 O are held together by hydrogen bonds. • Giving water a significantly higher meting point compared to other Group 6 hydrides • Hydrogen bonding makes ice less dense than water
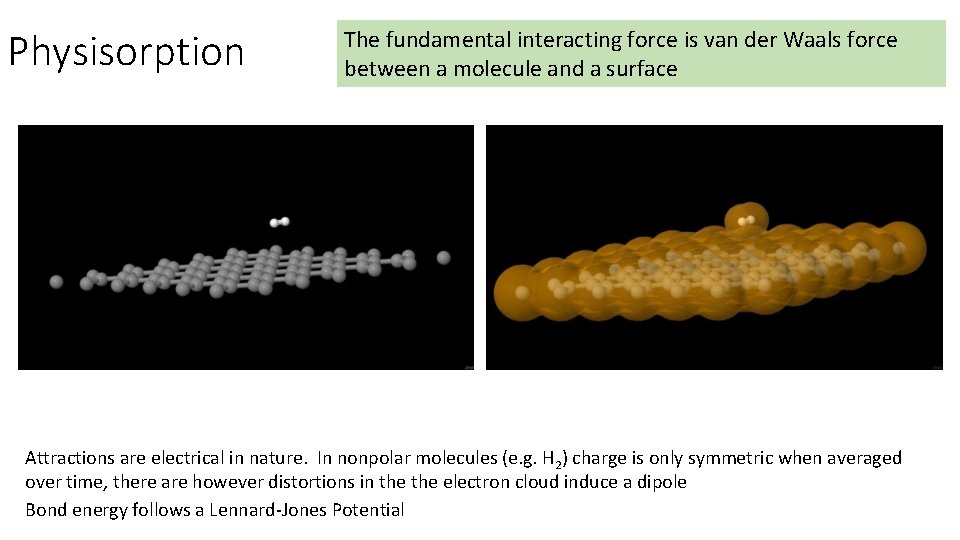
Physisorption The fundamental interacting force is van der Waals force between a molecule and a surface Attractions are electrical in nature. In nonpolar molecules (e. g. H 2) charge is only symmetric when averaged over time, there are however distortions in the electron cloud induce a dipole Bond energy follows a Lennard-Jones Potential

Physisorption – hydrogen storage Mg 2 Ni. H 4 La. Ni 5 H 5 Properties of matter week 3 H 2 (Liquid) H 2 (200 bar)
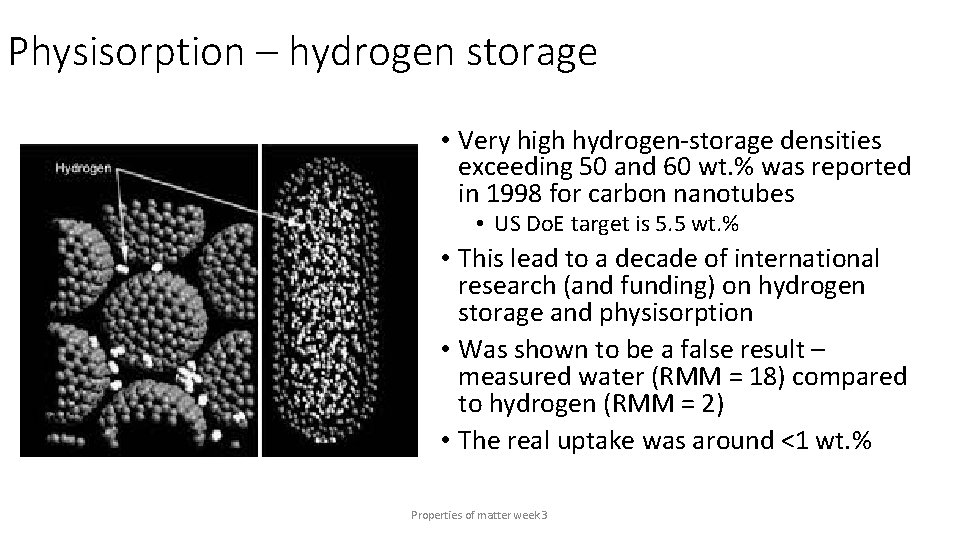
Physisorption – hydrogen storage • Very high hydrogen-storage densities exceeding 50 and 60 wt. % was reported in 1998 for carbon nanotubes • US Do. E target is 5. 5 wt. % • This lead to a decade of international research (and funding) on hydrogen storage and physisorption • Was shown to be a false result – measured water (RMM = 18) compared to hydrogen (RMM = 2) • The real uptake was around <1 wt. % Properties of matter week 3
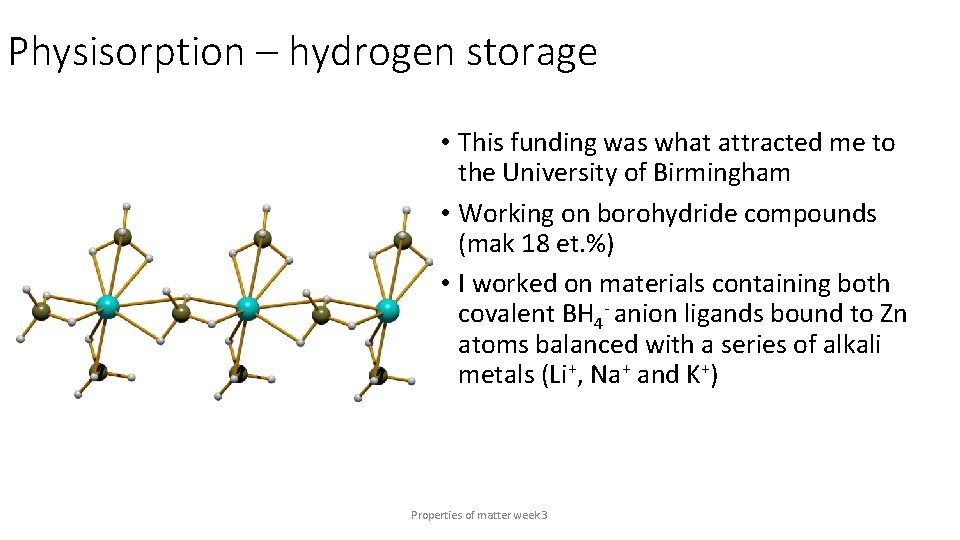
Physisorption – hydrogen storage • This funding was what attracted me to the University of Birmingham • Working on borohydride compounds (mak 18 et. %) • I worked on materials containing both covalent BH 4 - anion ligands bound to Zn atoms balanced with a series of alkali metals (Li+, Na+ and K+) Properties of matter week 3
- Slides: 30