Properties of Matter are either Extensive depend on













- Slides: 21


Properties of Matter are either… • Extensive depend on the amount of matter present (such as mass, volume, and the amount of energy of a substance) OR • Intensive do not depend on the amount of matter present (such as melting point, boiling point, or density)

Properties of Matter • Physical Properties Characteristics of matter that can be observed or measured without changing the identity of the substance. • Chemical Properties Property that relates to a substance’s ability to undergo changes that transform it into different substances.

Density • A physical property of matter that can be used to identify a substance. • Density = mass volume Mass is the amount of matter in an object (g or kg, etc. ) Volume is the amount of space occupied by an object. (m. L or cm 3, etc. )

Example Problems: Write the problem, the solve… • What is the density of a metal that has a mass of 72. 5 g and a volume of 14. 5 cm 3? • A block has dimensions of 0. 33 m x 465 mm x 6. 8 dm and a mass of 539 kg. What is its density in g/cm 3? • How many cubic centimeters are occupied by 85. 0 g of Zn if the density is 7. 14 g/cm 3? • The density of a solution is 1. 27 g/m. L. What is the mass of the 536 m. L solution?

States of Matter • The state of matter is a physical property. • There are four states of matter: Solid Liquid Gas Plasma

• Solids have a definite shape and volume • Particles are packed tightly together in a fixed position

• Liquids have a definite volume but no definite shape • Particles move more rapidly than in a solid and can move past one another

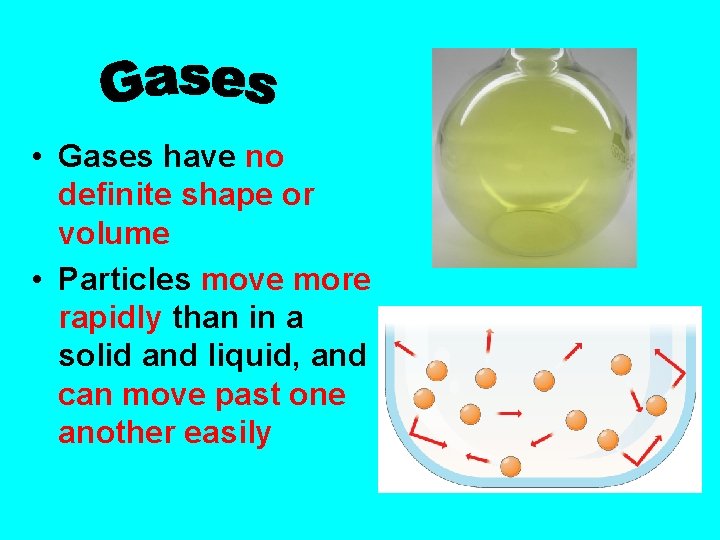
• Gases have no definite shape or volume • Particles move more rapidly than in a solid and liquid, and can move past one another easily

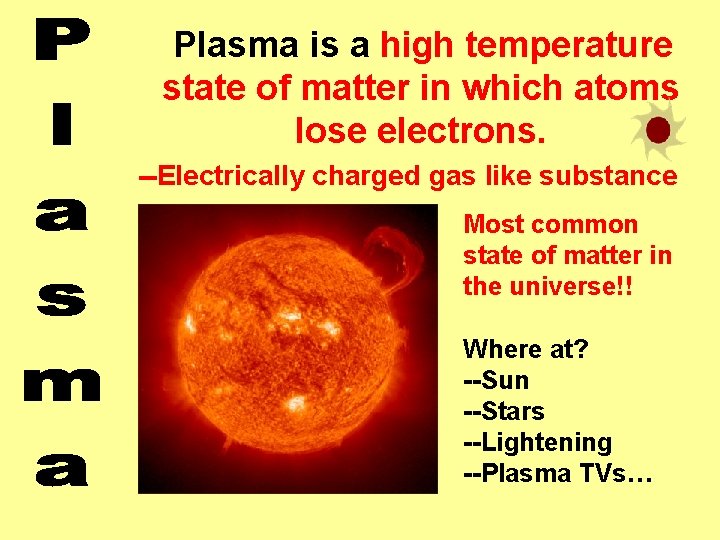
Plasma is a high temperature state of matter in which atoms lose electrons. --Electrically charged gas like substance Most common state of matter in the universe!! Where at? --Sun --Stars --Lightening --Plasma TVs…

Changes in Matter • Physical Changes – Change of a substance that does not involve a change a substance’s identity Tearing • Chemical Changes – Changes in which one or more substances in converted into a different substance (reacting, burning…)

• There are six phase changes or state changes that occur. All are physical changes in matter: Sublimation Evaporation Vaporization Melting Freezing Condensation

• Vaporization • Melting is the “boiling” is the changing of a liquid solid into a gas at the liquid boiling point. • Ex. Water boiling at 100 degrees Celsius

• Sublimation is • Evaporation is the changing of a liquid when a solid into a gas below a changes into a substance’s boiling gas without point. going through • Ex. Water the liquid state. evaporating in the • Ex. Dry ice sun at 75 degrees Celsius

Freezing is the changing of a liquid into a solid. Condensation is the changing of a gas into a liquid.

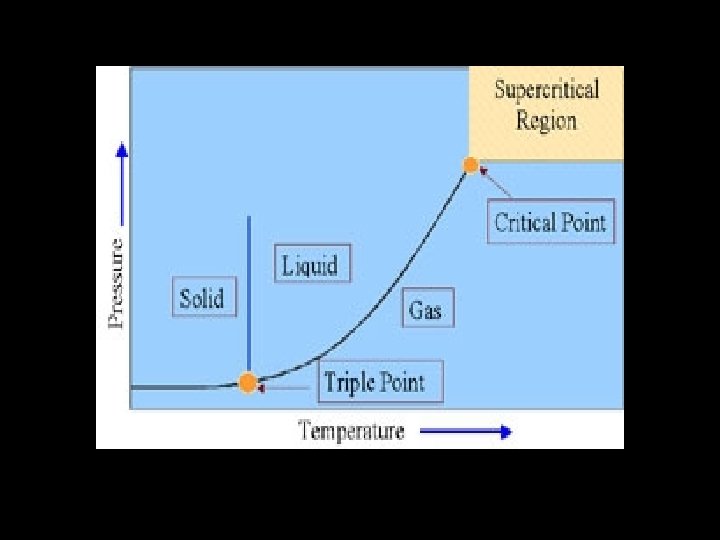
Phase Diagrams Phase diagrams can be used to show the conditions under which the phases of a substance exist.


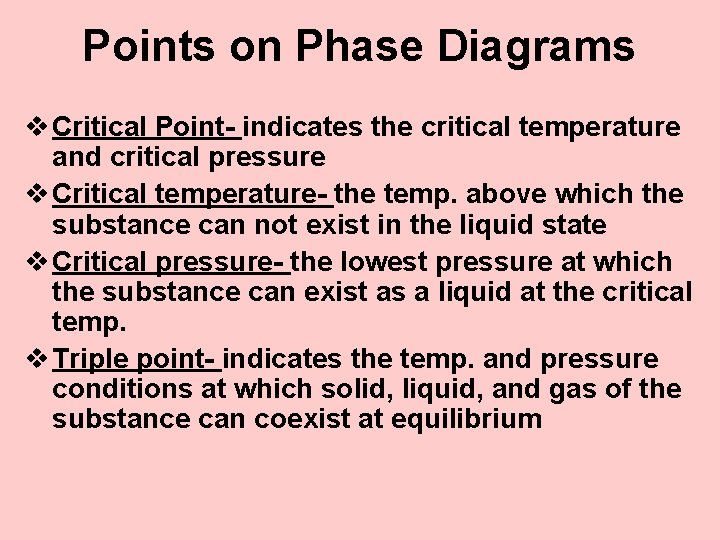
Points on Phase Diagrams v Critical Point- indicates the critical temperature and critical pressure v Critical temperature- the temp. above which the substance can not exist in the liquid state v Critical pressure- the lowest pressure at which the substance can exist as a liquid at the critical temp. v Triple point- indicates the temp. and pressure conditions at which solid, liquid, and gas of the substance can coexist at equilibrium

Chemical Changes …are changes in which one or more substances are converted into different substances

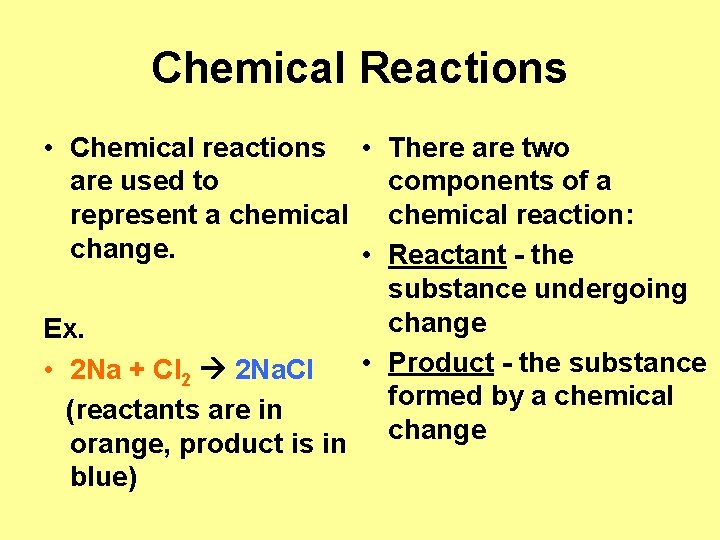
Chemical Reactions • Chemical reactions • There are two are used to components of a represent a chemical reaction: change. • Reactant - the substance undergoing change Ex. • Product - the substance • 2 Na + Cl 2 2 Na. Cl formed by a chemical (reactants are in change orange, product is in blue)

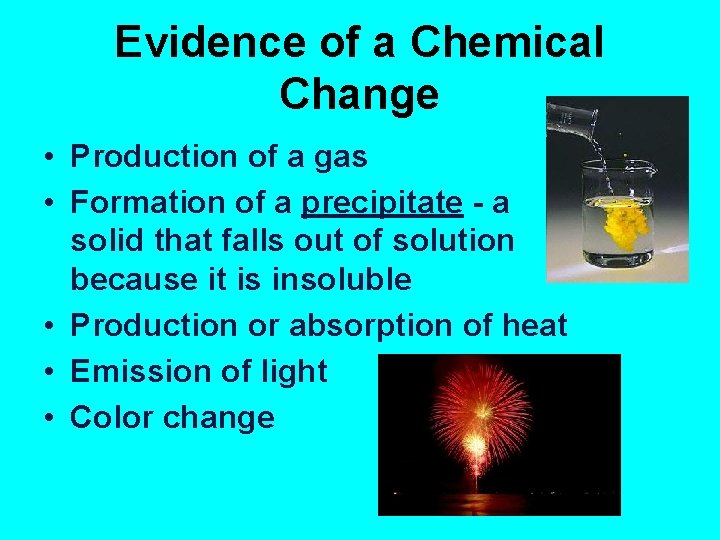
Evidence of a Chemical Change • Production of a gas • Formation of a precipitate - a solid that falls out of solution because it is insoluble • Production or absorption of heat • Emission of light • Color change