Properties of Matter 2016 Matter is anything that




- Slides: 9

Properties of Matter (2016) Matter is anything that has mass and volume Property is a characteristic

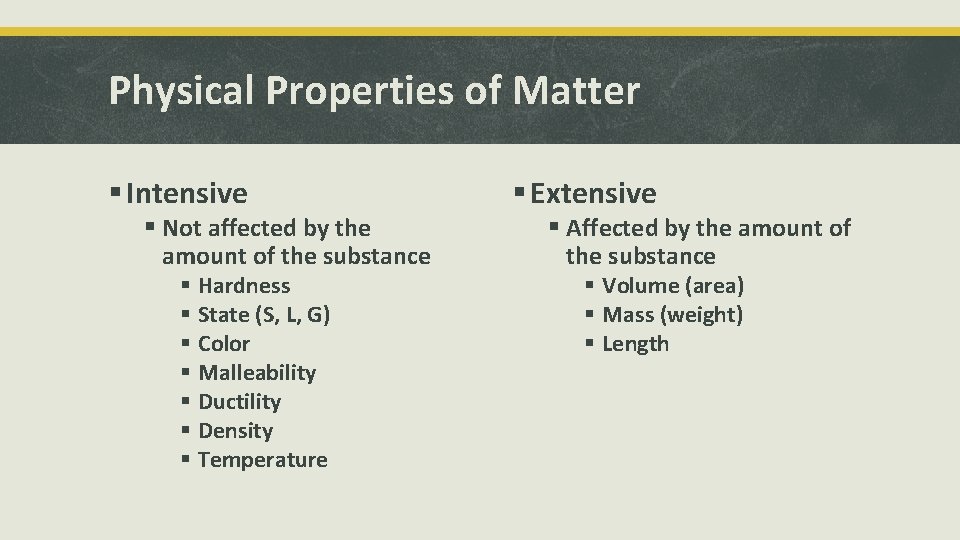
Physical Properties of Matter § Intensive § Not affected by the amount of the substance § Hardness § State (S, L, G) § Color § Malleability § Ductility § Density § Temperature § Extensive § Affected by the amount of the substance § Volume (area) § Mass (weight) § Length

Definitions § Malleable (capable of being hammered, rolled or pressed into various shapes without being broken) § Ductile (able to be drawn into a wire) § Mass (amount of matter in an object, measured with a balance) § Weight (effect of gravity, therefore differs with location) § Density = Mass/Volume

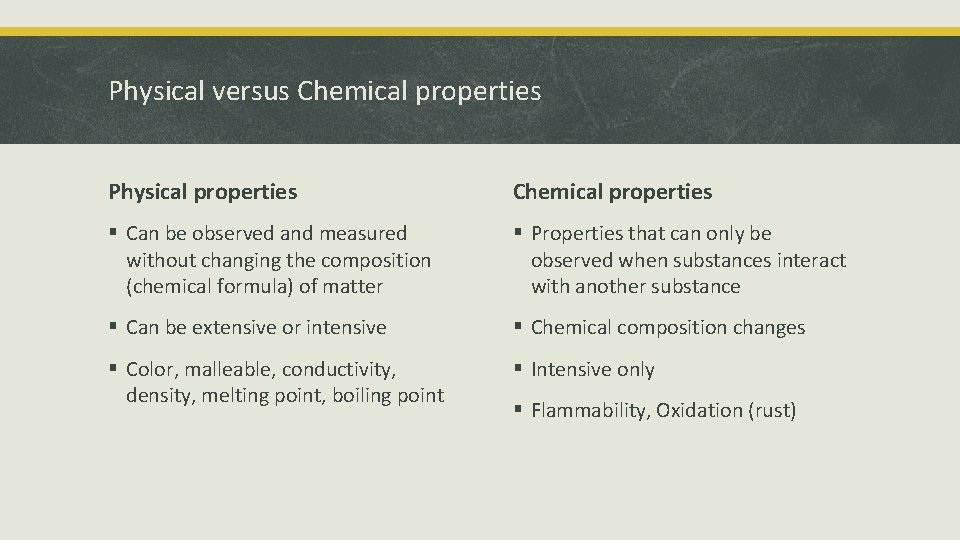
Physical versus Chemical properties Physical properties Chemical properties § Can be observed and measured without changing the composition (chemical formula) of matter § Properties that can only be observed when substances interact with another substance § Can be extensive or intensive § Chemical composition changes § Color, malleable, conductivity, density, melting point, boiling point § Intensive only § Flammability, Oxidation (rust)

Evidence that a chemical reaction may have occurred § Gas production § Formation of a solid § Heat or light is given off § Change in color § Odor is produced

States of Matter Solid, liquid or gas

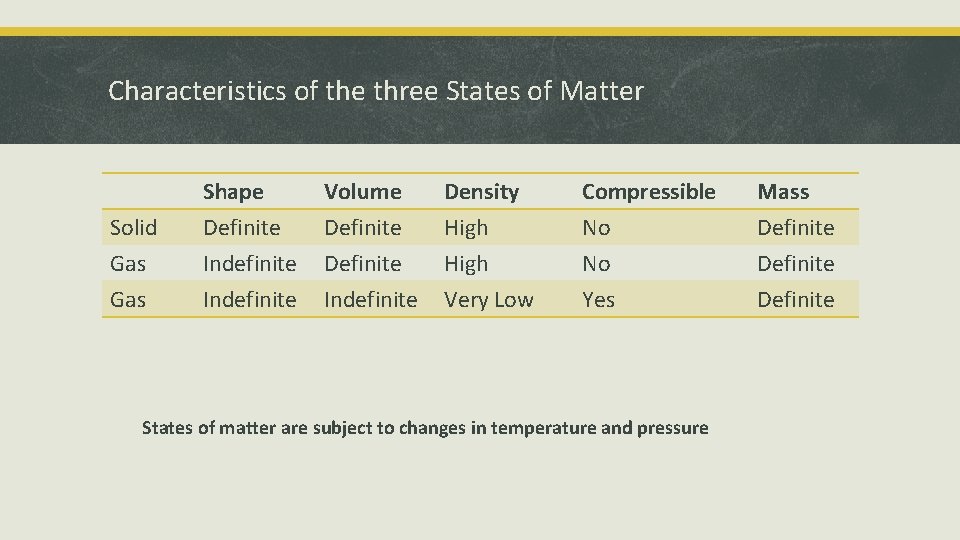
Characteristics of the three States of Matter Solid Gas Shape Definite Indefinite Volume Definite Indefinite Density High Very Low Compressible No No Yes States of matter are subject to changes in temperature and pressure Mass Definite

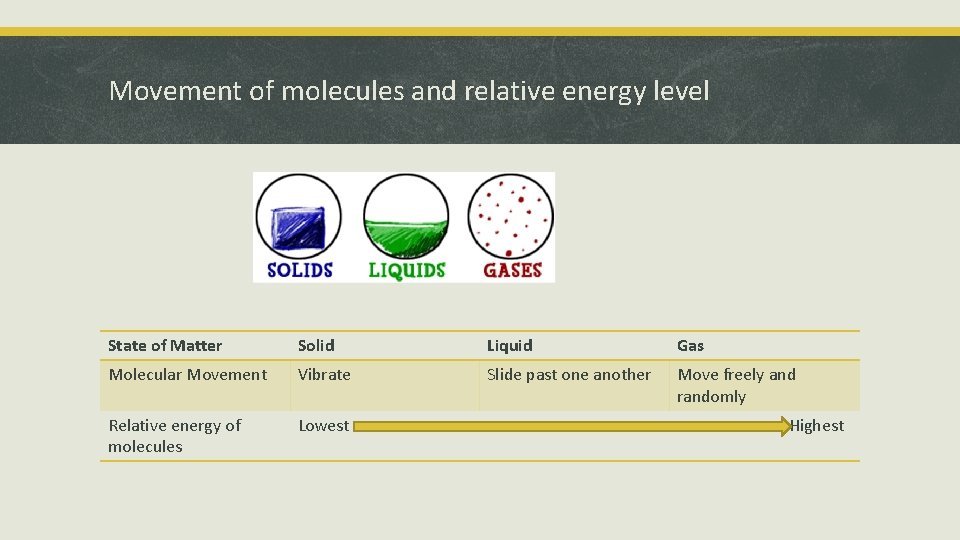
Movement of molecules and relative energy level State of Matter Solid Liquid Gas Molecular Movement Vibrate Slide past one another Move freely and randomly Relative energy of molecules Lowest Highest

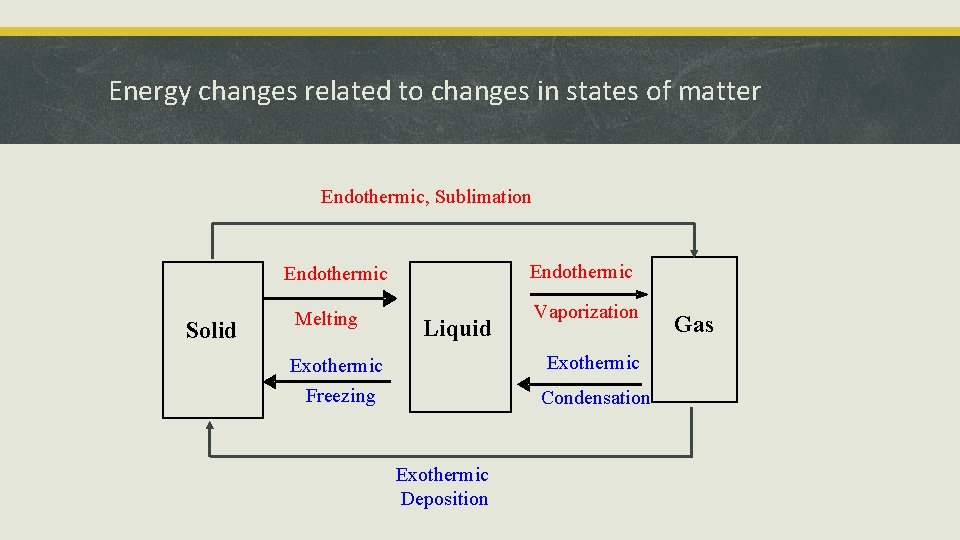
Energy changes related to changes in states of matter Endothermic, Sublimation Endothermic Solid Melting Liquid Vaporization Exothermic Freezing Condensation Exothermic Deposition Gas

















