Properties of Magma Chapter 7 Section 2 Elements









- Slides: 10

Properties of Magma Chapter 7 Section 2

Elements & Compounds n Element: Substance that cannot be broken down into other substances – Examples: Carbon, Hydrogen, Oxygen n Compound: Substance made up of two or more elements that have been chemically combined – Examples: Water, Carbon Dioxide, Table Salt

Physical and Chemical Properties n Each substance has a particular physical and chemical properties n These properties used to identify a substance n Physical and chemical properties are also used to predict how a substance will behave

Physical Properties n Any characteristic that does not alter the atomic or chemical properties of a substance n Examples of Physical Properties – Density – Hardness – Melting and boiling point – Magnetic

Chemical Properties n Any property that produces a change in the composition and results in a new substance being created – Ex) Metal Rusting or Wood Burning n Evidence of Chemical Reactions – – – Smoke Color Change Flames Bubbles or Fizzing Production of a Solid

What is Viscosity? n Liquids can flow freely from place to place n Viscosity: n Each resistance of a liquid to flowing liquids differ in viscosity, causing some liquids to flow more easily than others

What is Viscosity? n The greater the viscosity of a liquid, the slower it flows – Ex) Honey n The lower the viscosity, the faster it flows – Ex) Water, Rubbing Alcohol, Vinegar n Each substance has different viscosities determined by the movement of particles that make up each liquid

Viscosity of Magma n Viscosity of Magma varies of Magma is dependent on silica content and temperature

Magma and Silica Content n Silica is a compound made up of silicon and oxygen n The more silica magma contains, the higher the viscosity—light colored lava n The less silica magma contains, the lower the viscosity---dark colored lava

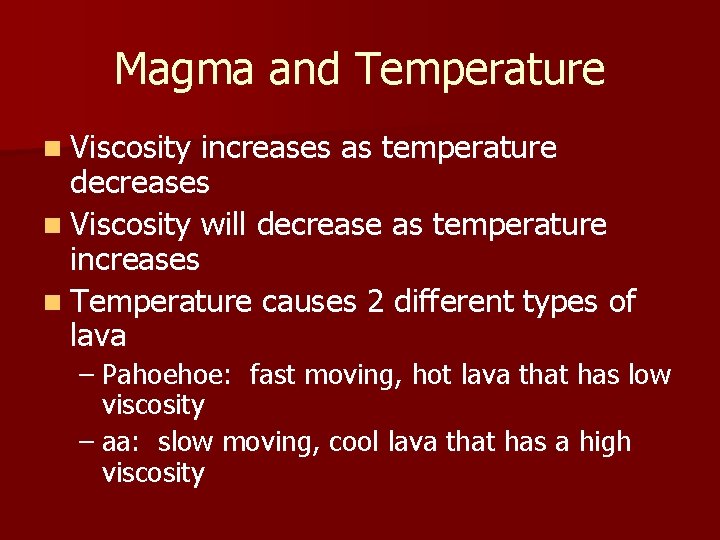
Magma and Temperature n Viscosity increases as temperature decreases n Viscosity will decrease as temperature increases n Temperature causes 2 different types of lava – Pahoehoe: fast moving, hot lava that has low viscosity – aa: slow moving, cool lava that has a high viscosity