Properties of Logarithms Lesson 5 5 Basic Properties
Properties of Logarithms Lesson 5. 5
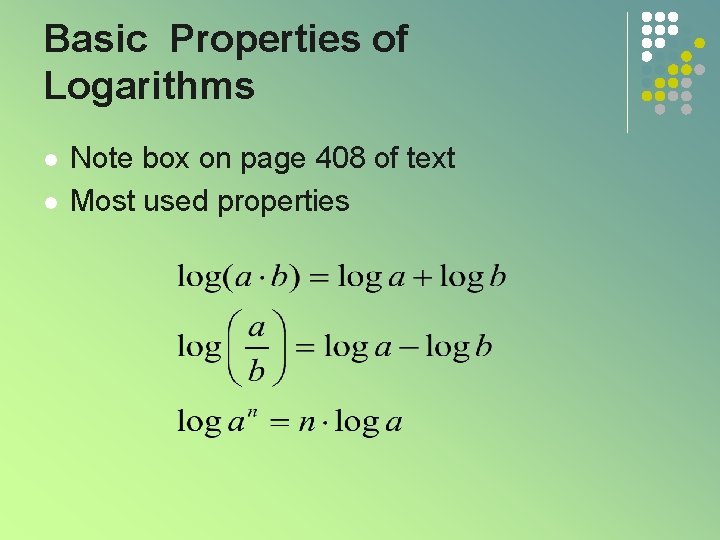
Basic Properties of Logarithms l l Note box on page 408 of text Most used properties
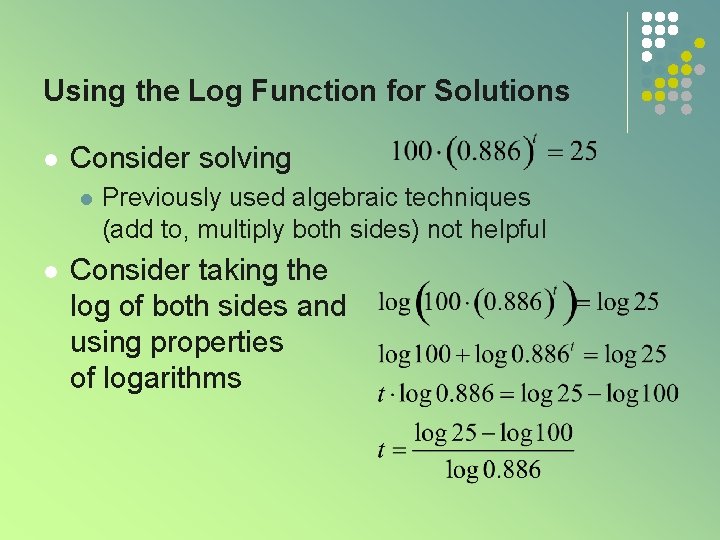
Using the Log Function for Solutions l Consider solving l l Previously used algebraic techniques (add to, multiply both sides) not helpful Consider taking the log of both sides and using properties of logarithms

Try It Out l Consider solution of 1. 7(2. 1) 3 x = 2(4. 5)x l Steps l l Take log of both sides Change exponents inside log to coefficients outside Isolate instances of the variable Solve for variable

Natural Logarithms l l We have used base of 10 for logs Another commonly used base for logs is e l l ) e has other interesting properties l l e is an irrational number (as is Later to be discovered in calculus Use ln button on your calculator
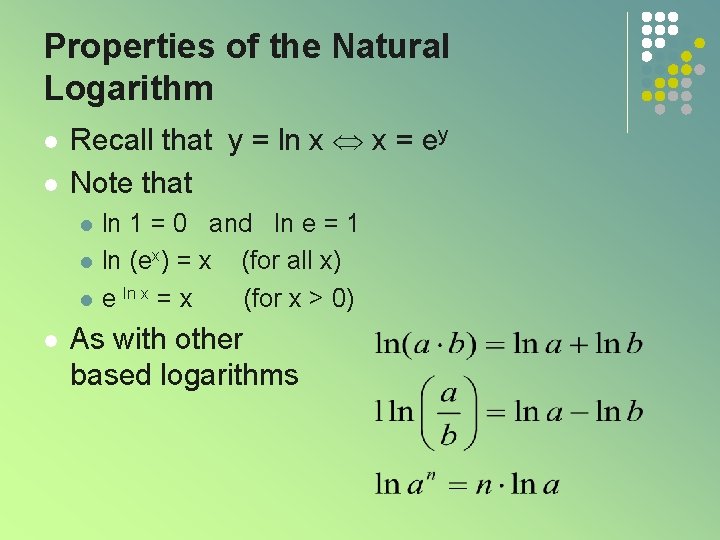
Properties of the Natural Logarithm l l Recall that y = ln x x = ey Note that l l ln 1 = 0 and ln e = 1 ln (ex) = x (for all x) e ln x = x (for x > 0) As with other based logarithms
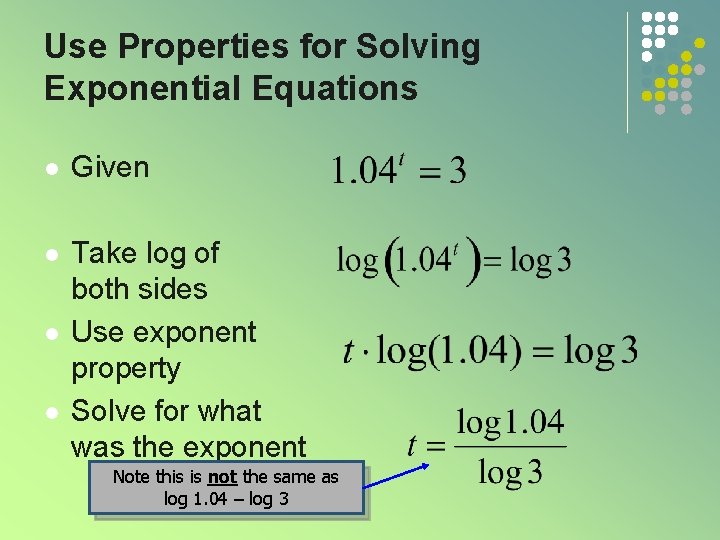
Use Properties for Solving Exponential Equations l Given l Take log of both sides Use exponent property Solve for what was the exponent l l Note this is not the same as log 1. 04 – log 3
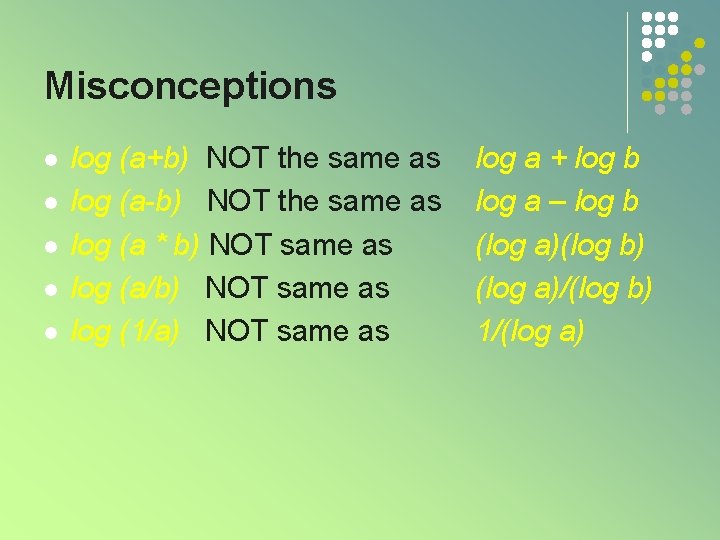
Misconceptions l l log (a+b) NOT the same as log (a-b) NOT the same as log (a * b) NOT same as log (a/b) NOT same as log (1/a) NOT same as log a + log b log a – log b (log a)(log b) (log a)/(log b) 1/(log a)
Usefulness of Logarithms l Logarithms useful in measuring quantities which vary widely l l l Acidity (p. H) of a solution Sound (decibels) Earthquakes (Richter scale)
![Chemical Acidity l p. H defined as l l l p. H = -log[H+] Chemical Acidity l p. H defined as l l l p. H = -log[H+]](http://slidetodoc.com/presentation_image_h/e4047c90fff5dcc5d11513869a200508/image-10.jpg)
Chemical Acidity l p. H defined as l l l p. H = -log[H+] where [H+] is hydrogen ion concentration measured in moles per liter If seawater is [H+]= 1. 1*10 -8 l then –log(1. 1*10 -8) = 7. 96

Chemical Acidity l What would be the hydrogen ion concentration of vinegar with p. H = 3?
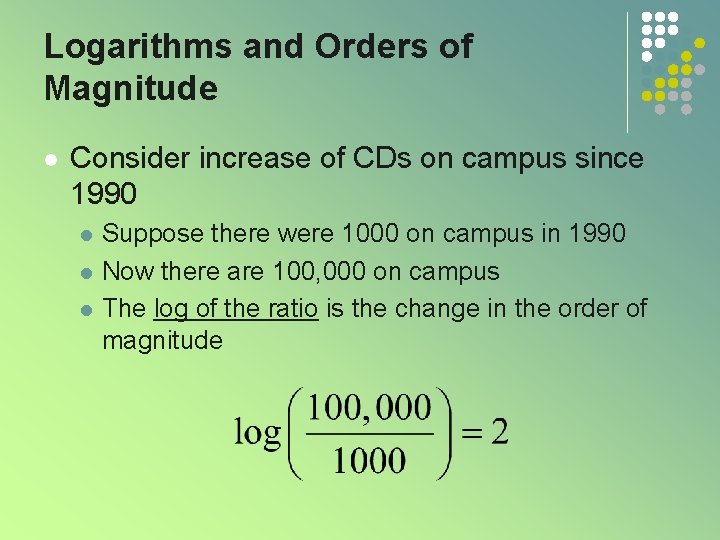
Logarithms and Orders of Magnitude l Consider increase of CDs on campus since 1990 l l l Suppose there were 1000 on campus in 1990 Now there are 100, 000 on campus The log of the ratio is the change in the order of magnitude

Decibels l Suppose I 0 is the softest sound the human ear can hear l measured in watts/cm 2 l And I is the watts/cm 2 of a given sound l Then the decibels of the sound is The log of the ratio

Logarithms and Orders of Magnitude l l We use the log function because it “counts” the number of powers of 10 This is necessary because of the vast range of sound intensity that the human ear can hear

Decibels l If a sound doubles, how many units does its decibel rating increase?
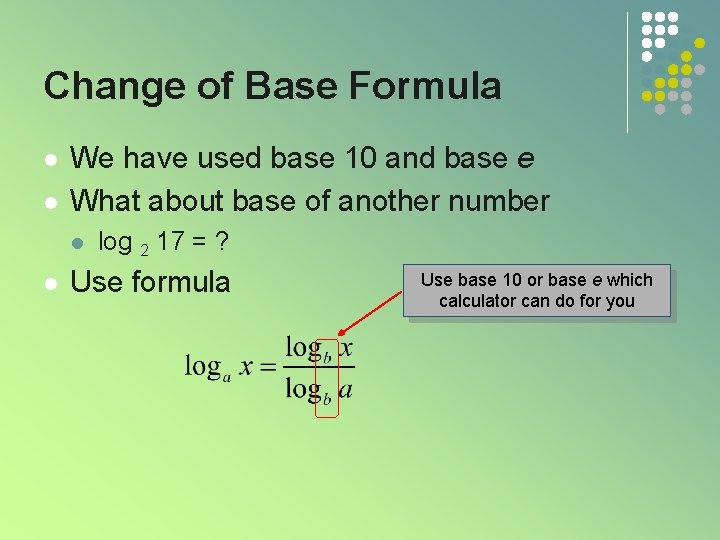
Change of Base Formula l l We have used base 10 and base e What about base of another number l l log 2 17 = ? Use formula Use base 10 or base e which calculator can do for you

Assignment l l l Lesson 5. 5 Page 414 Exercises 1 – 61 odd
- Slides: 17