Properties of Liquids Density and Buoyancy Definitions Density

Properties of Liquids: Density and Buoyancy

Definitions Density: The mass per unit volume of a material. Buoyancy: The ability of a fluid to exert an upward force on a floating object.
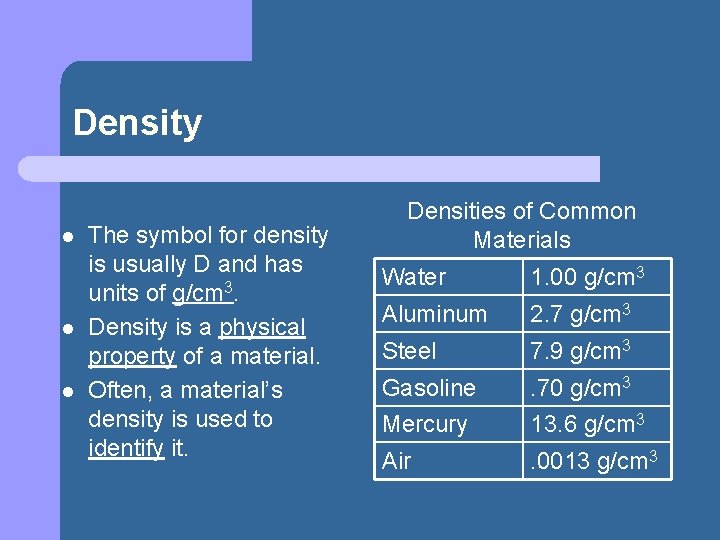
Density l l l The symbol for density is usually D and has units of g/cm 3. Density is a physical property of a material. Often, a material’s density is used to identify it. Densities of Common Materials Water 1. 00 g/cm 3 Aluminum 2. 7 g/cm 3 Steel 7. 9 g/cm 3 Gasoline . 70 g/cm 3 Mercury 13. 6 g/cm 3 Air . 0013 g/cm 3
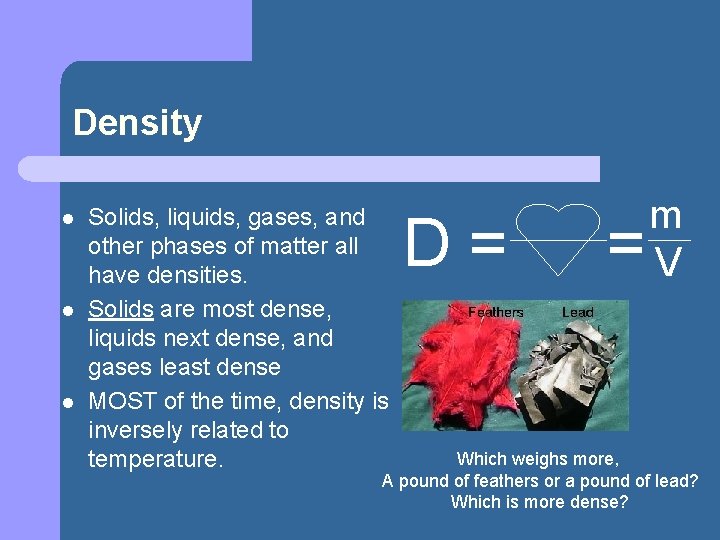
Density l l l Solids, liquids, gases, and other phases of matter all have densities. Solids are most dense, liquids next dense, and gases least dense MOST of the time, density is inversely related to temperature. D= = m V Which weighs more, A pound of feathers or a pound of lead? Which is more dense?
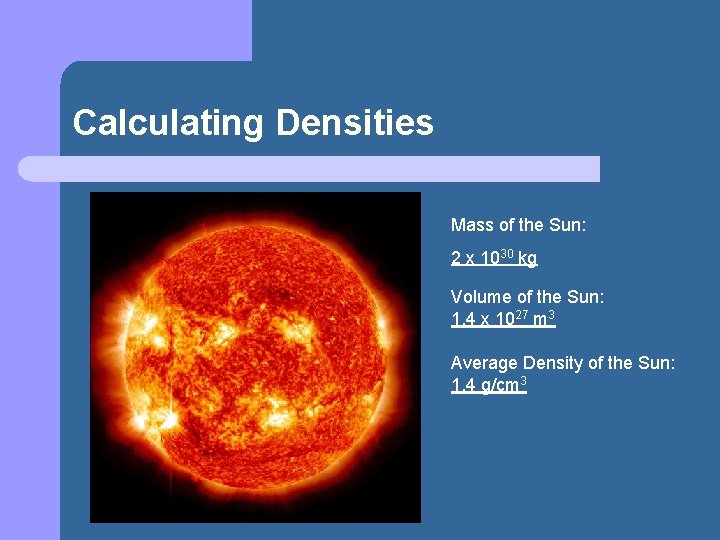
Calculating Densities Mass of the Sun: 2 x 1030 kg Volume of the Sun: 1. 4 x 1027 m 3 Average Density of the Sun: 1. 4 g/cm 3
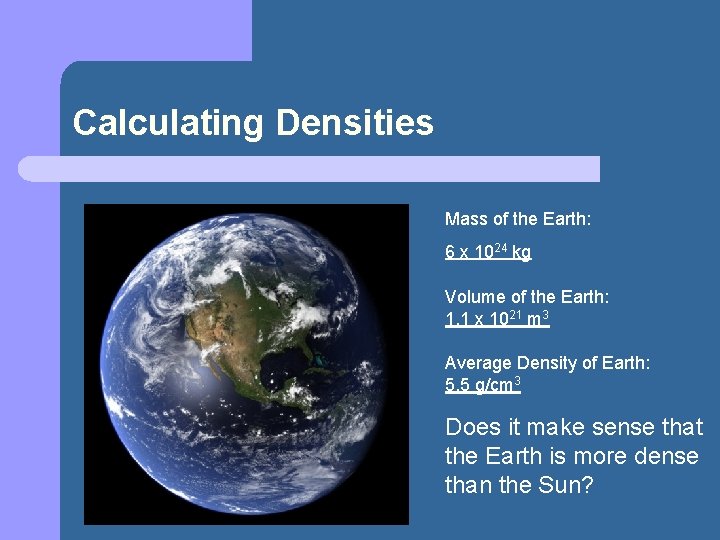
Calculating Densities Mass of the Earth: 6 x 1024 kg Volume of the Earth: 1. 1 x 1021 m 3 Average Density of Earth: 5. 5 g/cm 3 Does it make sense that the Earth is more dense than the Sun?

A Density Problem: Does this help? l l l You found this ring on the track at your school. You wondered what it was made of. You calculated it’s density to be approximately 7. 9 g/cm 3. What is it made of? Metal Density g/cm 3 Aluminum 2. 70 Gold 19. 30 Copper 8. 63 Steel 7. 87 Silver 10. 40 Chromium 7. 10 Lead 11. 30

Forces Newton’s Laws: 1. ) The law of inertia: An object in motion stay in motion until it is acted upon. Or, an object at rest stays at rest until acted upon. 2. ) F = m*a 3. ) When two bodies interact by a force, the force on the first body is equal in magnitude and opposite in direction to the force on the second body.

Archimedes’ Principle The buoyant force exerted on an object submersed in a fluid is equal to the weight of the fluid displaced.
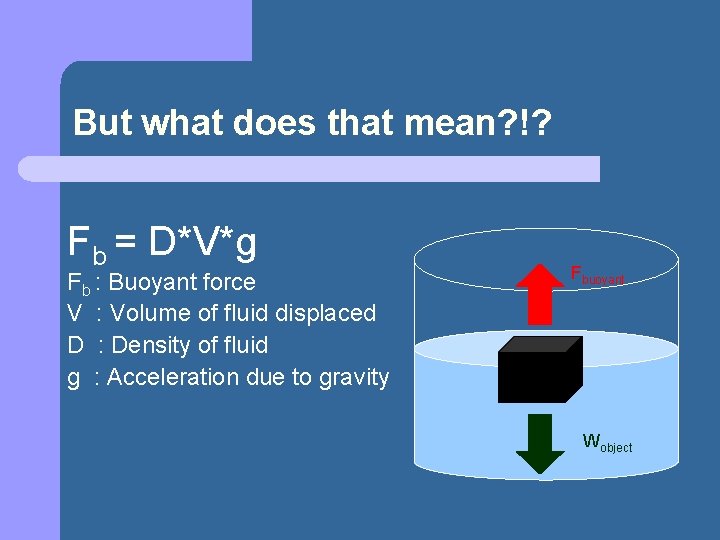
But what does that mean? !? Fb = D*V*g Fb : Buoyant force V : Volume of fluid displaced D : Density of fluid g : Acceleration due to gravity Fbuoyant Wobject
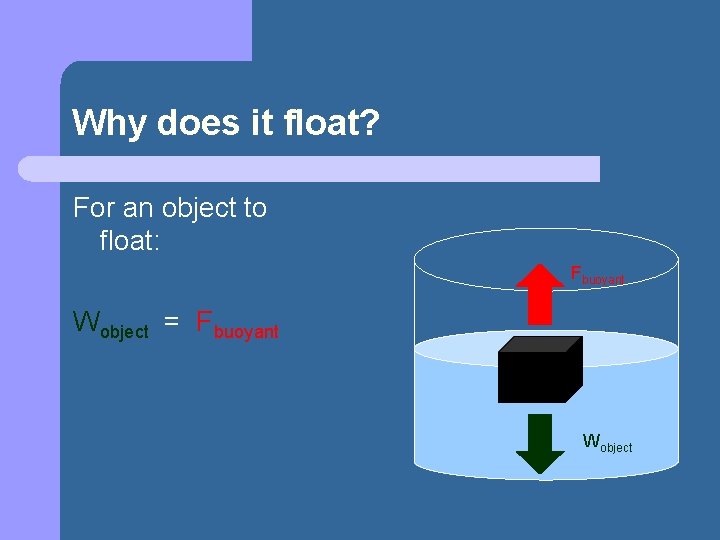
Why does it float? For an object to float: Fbuoyant Wobject = Fbuoyant Wobject

Buoyancy Calculations The polar bear on ice: A 500 kg polar bear sees a slab of ice floating by and tries to hop on. The ice has a volume of 4 m 3 and a density of. 92 g/cm 3. Seawater has a density of 1. 035 g/cm 3. Does the polar bear float away happily? Not this time! Fb = 4140 N < Wbear = 4900 N

Activity l You will determine the density of a liquid and use that density to identify the liquid. l Good luck!

Questions?
- Slides: 14