Properties of Ionic and Molecular Compounds Solubility and

Properties of Ionic and Molecular Compounds Solubility and Properties of Water Section 2. 3
Objectives �Classify ionic and molecular compounds based on their properties �Use a solubility chart to predict whether an ionic compound is soluble in water �Relate the molecular structure of water to its properties

Solubility �Solubility: the ability to dissolve (in our case, in water) �Aqueous (aq) : when something is very soluble �Solid (s) : when something is slightly soluble/doesn’t dissolve well �Decide this by using a solubility table: �page 57 in textbook and on your data sheet
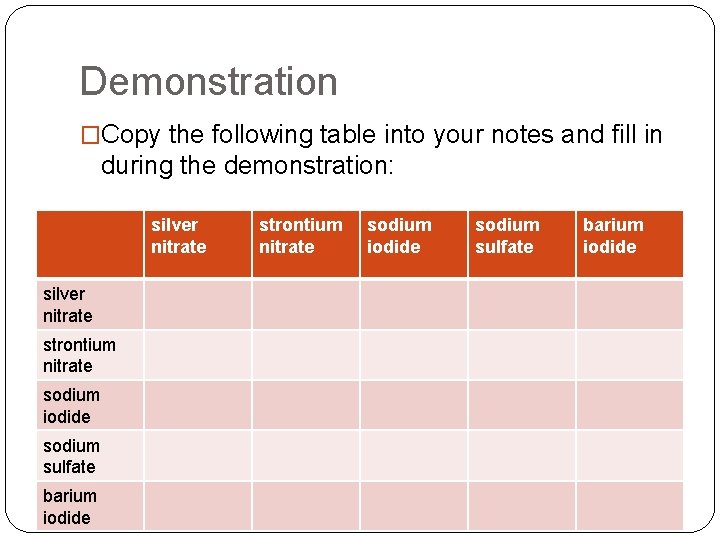
Demonstration �Copy the following table into your notes and fill in during the demonstration: silver nitrate strontium nitrate sodium iodide sodium sulfate barium iodide
How to use a solubility table � page 57 in textbook and on your data sheet �Break the compound up into its ions: �The negative ions (anions) are across the top �Find your negative ion and then look down into the chart to find the positive ion �“all” : all compounds with those ions �“most” : most compounds with those ions �“only with” : only compounds containing what’s listed �Which elements are group 1? Group 2?
Solubility Table � Find the solubility/state of the following compounds using the solubility table: �(NH 4)2 S �Ag. Cl �Pb. SO 4 �Sr(OH)2 �Fe(OH)3 �Au(NO 3)3 �Ag. CH 3 COO �potassium carbonate �iron (III) nitrate �ammonium sulfite �lead (IV) bromide
Demo Results �Which compounds produced solids? �Could we have predicted which ones would form solids? �Can you identify which compound made the precipitate? How? �What ions do I have? How will they recombine?
Properties of Water �Water is polar �Has a positive (hydrogen) and a negative (oxygen) end �Bent shape and unequal sharing of electrons �What does this mean can happen? �Water molecules are attracted to one another �Because polar, has several important properties: �Boils at higher temp �Why is this important? Hint: Think of how much of the earth is covered in water �Bodies of water serve to regulate temperatures (absorb heat during the summer and release in winter)

Formation of Ice �Is ice more or less dense than liquid water? �How do you know? �Water molecules spread out as it freezes making six-sided rings �Look at a snowflake: how many points does it have? �Space in between water molecules �Makes ice less dense �Why is this important? What might be affected if ice didn’t float?
- Slides: 9