Properties of Ionic and Covalent Bonds Ionic Compounds

Properties of Ionic and Covalent Bonds
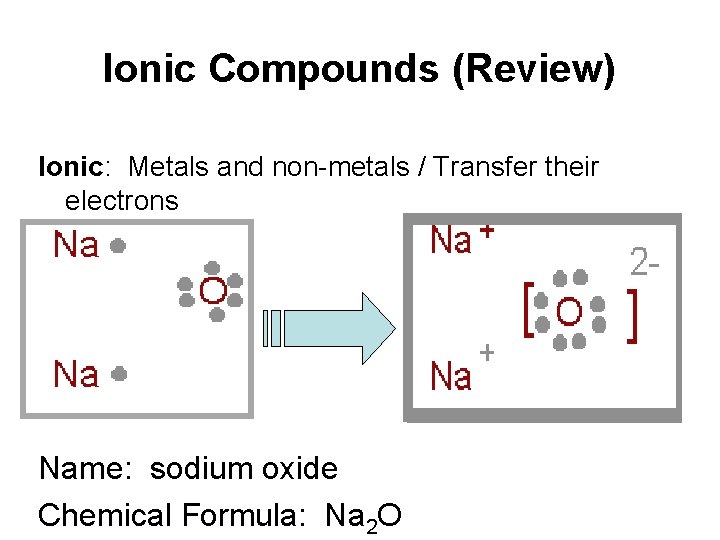
Ionic Compounds (Review) Ionic: Metals and non-metals / Transfer their electrons Name: sodium oxide Chemical Formula: Na 2 O

Properties of Ionic Compounds • Strong attraction between oppositely charged ions (cations and anions) – strong bond • High melting point • Conduct electricity when dissolved in water • Hard • Very little smell • Soluble in water
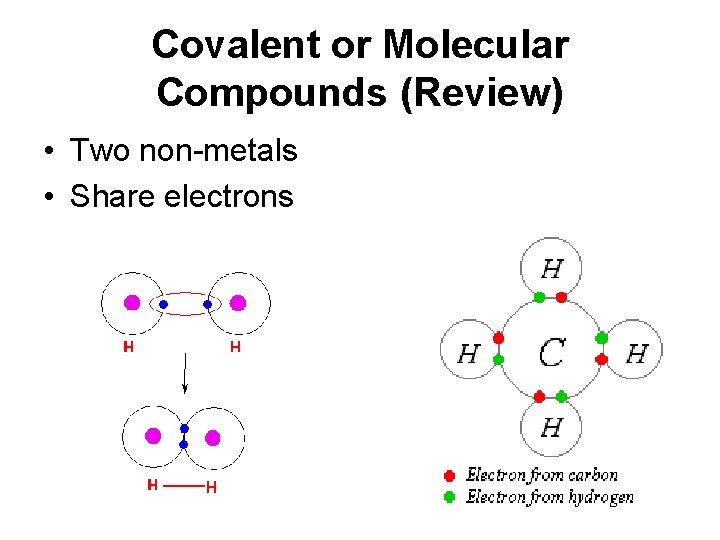
Covalent or Molecular Compounds (Review) • Two non-metals • Share electrons

Properties of Covalent Compounds • Bonds are strong but attraction between shared electrons is weak • Low melting points • Do not conduct electricity • Soft • Often have a scent • Not easily dissolved in water
Electrolytes • Electrolyte: a substance that when it is dissolved in water (aqueous solution) can conduct electricity. – Ionic compounds in aqueous solutions are electrolytes – Molecular compounds in aqueous solutions are considered to be non-electrolytes.

Major electrolytes in your body • • sodium (Na+) potassium (K+) chloride (Cl-) calcium (Ca 2+) magnesium (Mg 2+ ) bicarbonate (HCO 3 -) phosphate (PO 42 -) sulfate (SO 42 -)

• In humans, electrolytes help with a number of physical and vital processes. • Many heart and nerve functions, muscle control and coordination, and the body's ability to absorb fluids all depend on a healthy balance of electrolytes. • Many sports drinks contain added potassium and sodium to help restore the body's proper electrolyte balance after intense physical exertion.

• Notes
- Slides: 9