Properties of Fluids SPH 4 C Fluids Liquids


















- Slides: 31

Properties of Fluids SPH 4 C

Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container.

Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. If the fluids are at rest, the study of them is called _____. If the fluids are in motion, the study of them is called ______.

Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. If the fluids are at rest, the study of them is called fluid statics. If the fluids are in motion, the study of them is called ______.

Fluids Liquids and gases are both fluids: a fluid is any substance that flows and takes the shape of its container. If the fluids are at rest, the study of them is called fluid statics. If the fluids are in motion, the study of them is called fluid dynamics.

Fluids The science and technology of the mechanical properties of liquids is called _____. Similarly, the science and technology of the mechanical properties of air and other gases is called ______.

Fluids The science and technology of the mechanical properties of liquids is called hydraulics. Similarly, the science and technology of the mechanical properties of air and other gases is called ______.

Fluids The science and technology of the mechanical properties of liquids is called hydraulics. Similarly, the science and technology of the mechanical properties of air and other gases is called pneumatics.

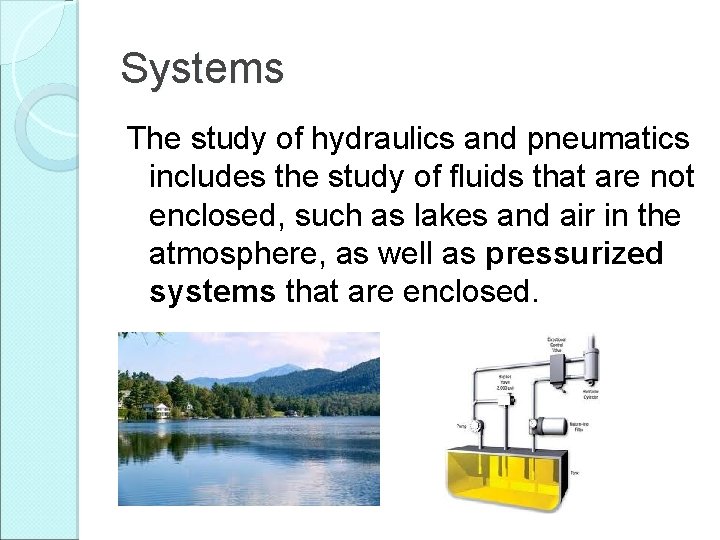
Systems The study of hydraulics and pneumatics includes the study of fluids that are not enclosed, such as lakes and air in the atmosphere, as well as pressurized systems that are enclosed.

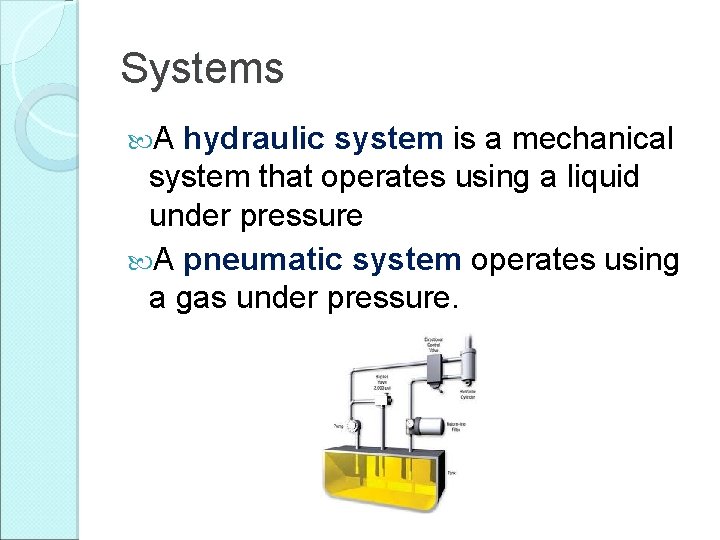
Systems A hydraulic system is a mechanical system that operates using a liquid under pressure A pneumatic system operates using a gas under pressure.

What’s the difference? Liquids flow and take the shape of their container but maintain a constant volume.

What’s the difference? Gases expand to fill the available volume.

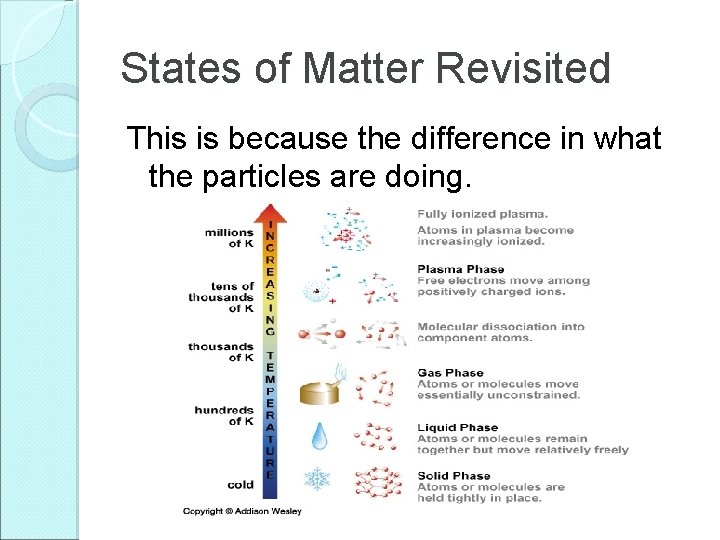
States of Matter Revisited This is because the difference in what the particles are doing.

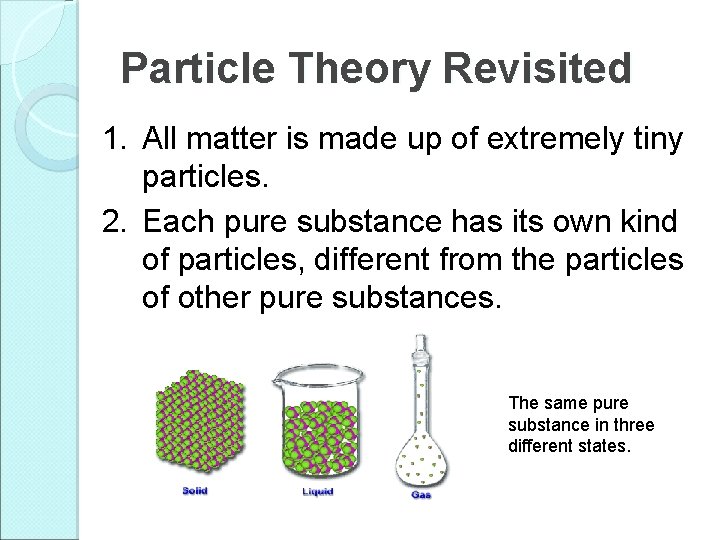
Particle Theory Revisited 1. All matter is made up of extremely tiny particles. 2. Each pure substance has its own kind of particles, different from the particles of other pure substances. The same pure substance in three different states.

Particle Theory Revisited 3. Particles are always moving. Particles at a higher temperature are generally moving faster on average than particles at a lower temperature. Remember the “Dance of the Molecules? ”

Particle Theory Revisited 4. Particles attract each other. See? They’re smiling.

Solids In a solid, the particles are moving slowly enough that this attraction keeps them in a rigid structure.

Liquids In a liquid, the particles move fast enough that they can’t stay in a rigid structure but they still want to stay close by.

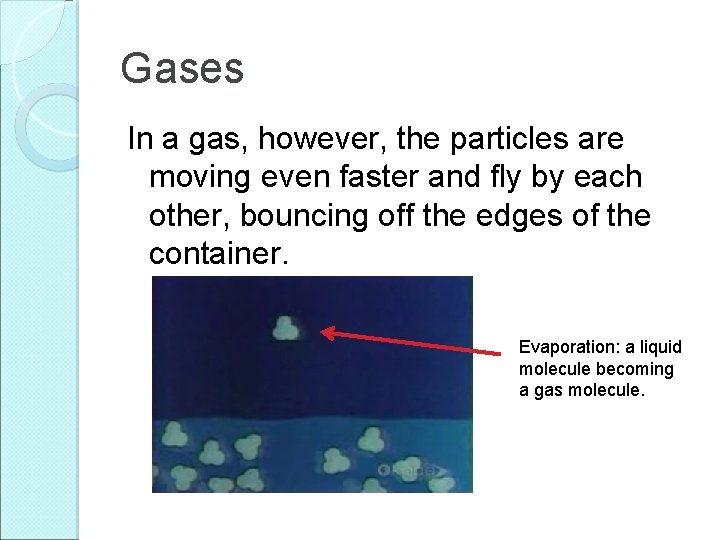
Gases In a gas, however, the particles are moving even faster and fly by each other, bouncing off the edges of the container. Evaporation: a liquid molecule becoming a gas molecule.

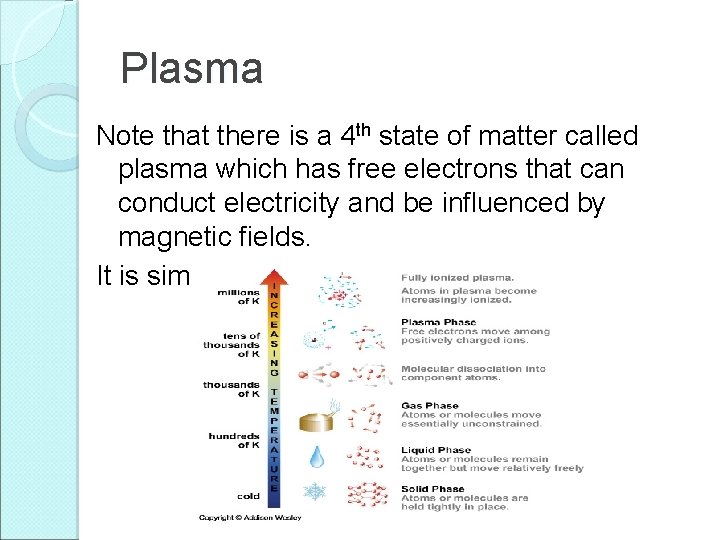
Plasma Note that there is a 4 th state of matter called plasma which has free electrons that can conduct electricity and be influenced by magnetic fields. It is similar to a gas in its properties.

Compressibility Gases are therefore highly compressible: their particles can be forced back closer together.

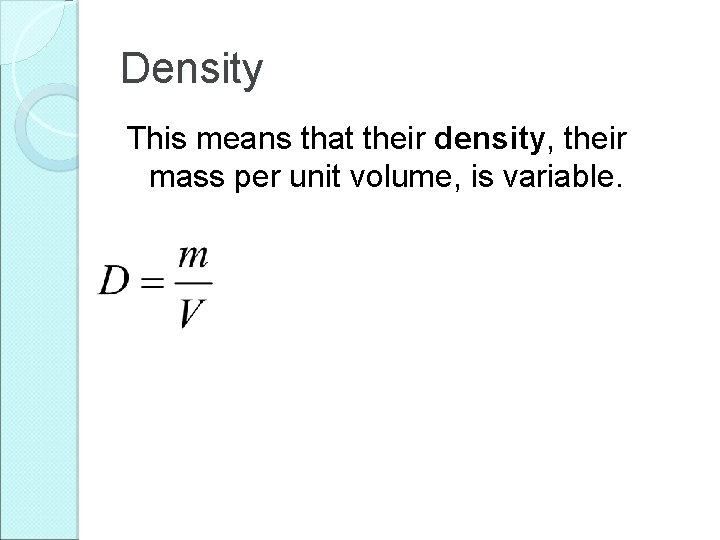
Density This means that their density, their mass per unit volume, is variable.

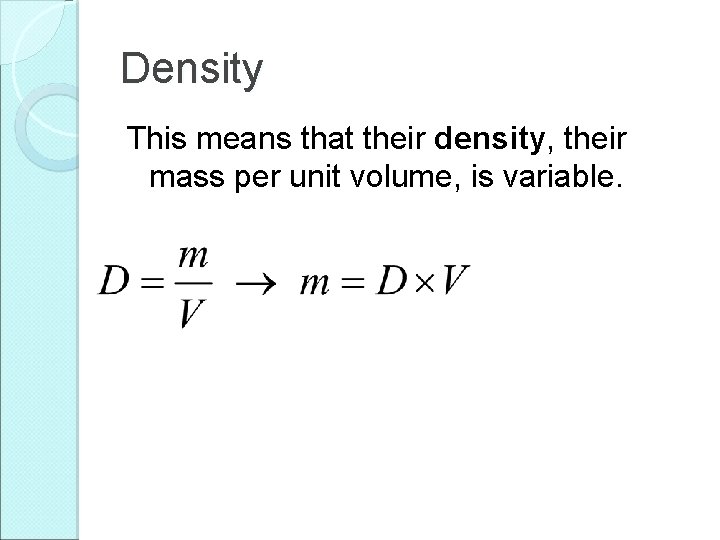
Density This means that their density, their mass per unit volume, is variable.

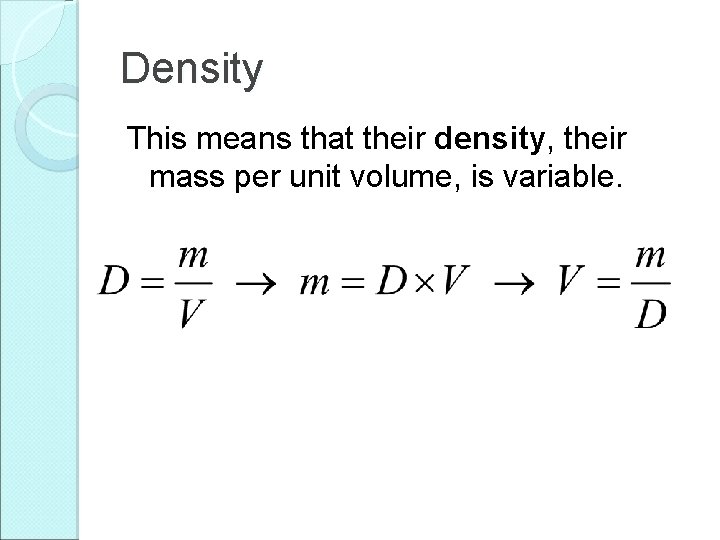
Density This means that their density, their mass per unit volume, is variable.

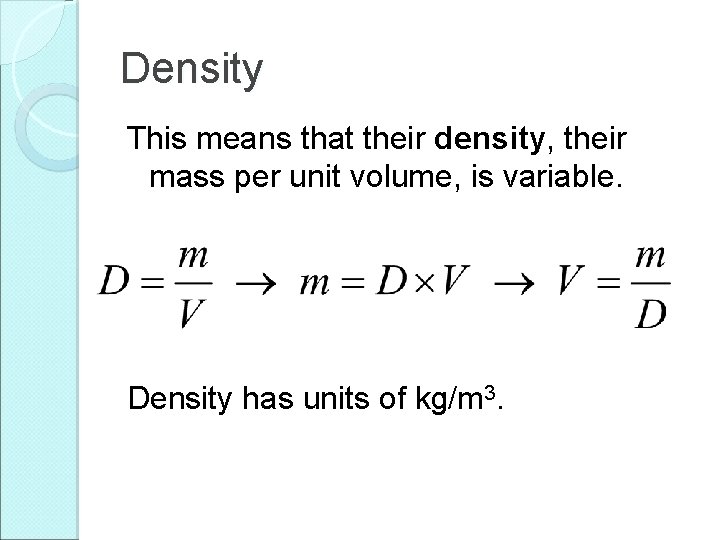
Density This means that their density, their mass per unit volume, is variable. Density has units of kg/m 3.

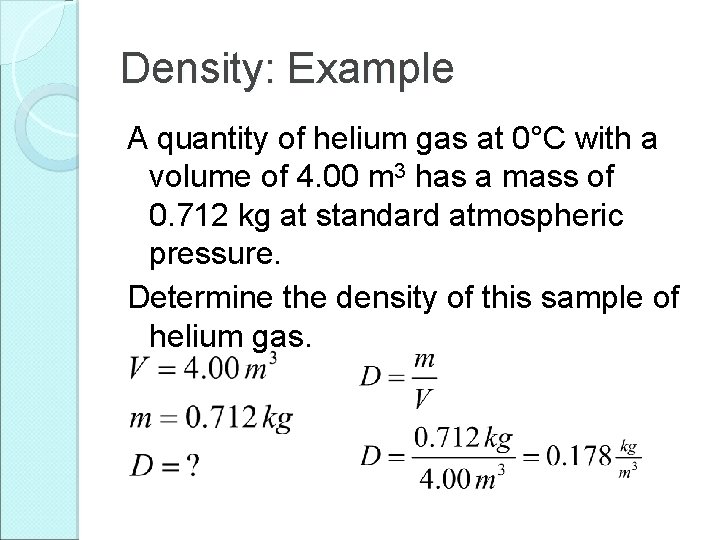
Density: Example A quantity of helium gas at 0°C with a volume of 4. 00 m 3 has a mass of 0. 712 kg at standard atmospheric pressure. Determine the density of this sample of helium gas.

Density: Example A quantity of helium gas at 0°C with a volume of 4. 00 m 3 has a mass of 0. 712 kg at standard atmospheric pressure. Determine the density of this sample of helium gas.

Density: Example A quantity of helium gas at 0°C with a volume of 4. 00 m 3 has a mass of 0. 712 kg at standard atmospheric pressure. Determine the density of this sample of helium gas.

Density: Example A quantity of helium gas at 0°C with a volume of 4. 00 m 3 has a mass of 0. 712 kg at standard atmospheric pressure. Determine the density of this sample of helium gas.

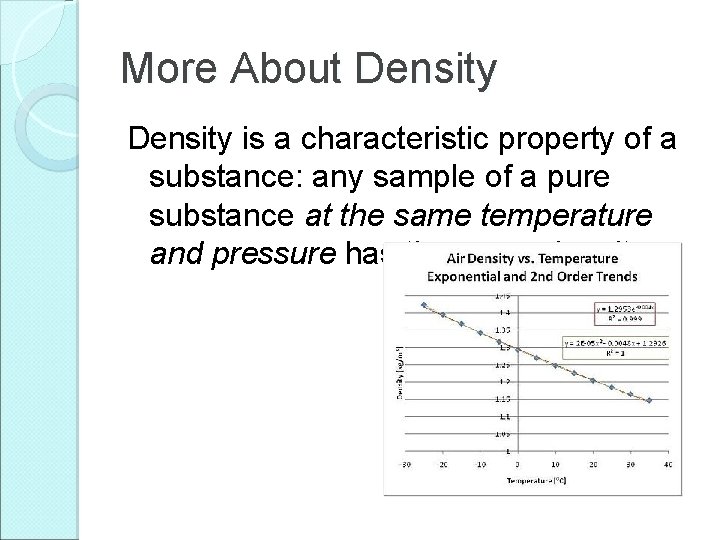
More About Density is a characteristic property of a substance: any sample of a pure substance at the same temperature and pressure has the same density.

More Practice The Density of Fluids Lab Activity
 Buoyancyability
Buoyancyability Netgear sph
Netgear sph Sph surface tension
Sph surface tension Sph +3
Sph +3 Sph cah toa
Sph cah toa Ohsu sph
Ohsu sph Sph saxion
Sph saxion Sph toon
Sph toon Sph 4 inches
Sph 4 inches Gas to solid
Gas to solid Properties of a solid
Properties of a solid Properties of solids liquids and gases
Properties of solids liquids and gases Properties of liquid state
Properties of liquid state Volumetric properties of pure fluids
Volumetric properties of pure fluids Physical properties of ice cube
Physical properties of ice cube Intensive vs extensive properties
Intensive vs extensive properties Venn diagram of liquid and gas
Venn diagram of liquid and gas Separation of immiscible liquids diagram
Separation of immiscible liquids diagram How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Frazier free water protocol printable
Frazier free water protocol printable Whats a physical change
Whats a physical change Plant growth with different liquids
Plant growth with different liquids Why are liquids incompressible
Why are liquids incompressible Flammable liquids
Flammable liquids Which is not a monophasic liquid dosage form
Which is not a monophasic liquid dosage form Examples of gas
Examples of gas Red liquid element
Red liquid element Soda is countable or uncountable
Soda is countable or uncountable Ka6o5vcprf8 -site:youtube.com
Ka6o5vcprf8 -site:youtube.com Chapter 19 liquids exercises answers
Chapter 19 liquids exercises answers Compressible and incompressible fluids
Compressible and incompressible fluids Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids