Properties of Emulsions and Foams FDSC 400 Goals










- Slides: 24

Properties of Emulsions and Foams FDSC 400

Goals • Properties of emulsions – Type – Size – Volume fraction • Destabilization of emulsions – Creaming – Flocculation – Coalescence • Foams

Emulsion A fine dispersion of one liquid in a second, largely immiscible liquid. In foods the liquids are inevitably oil and an aqueous solution.

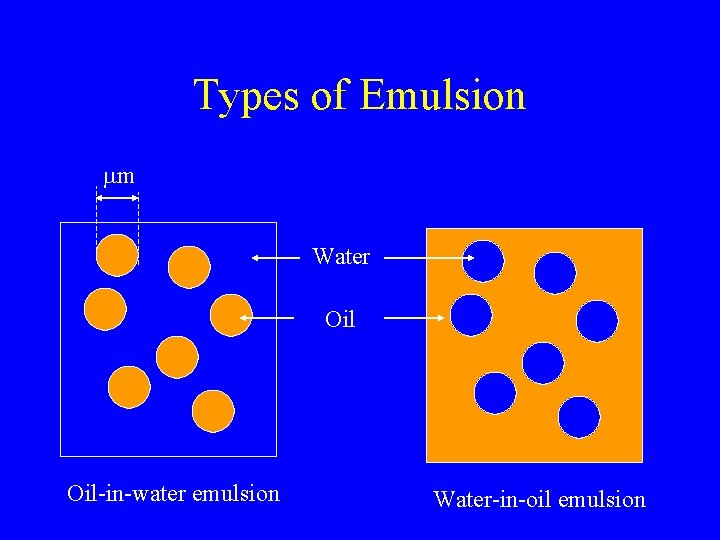
Types of Emulsion mm Water Oil-in-water emulsion Water-in-oil emulsion

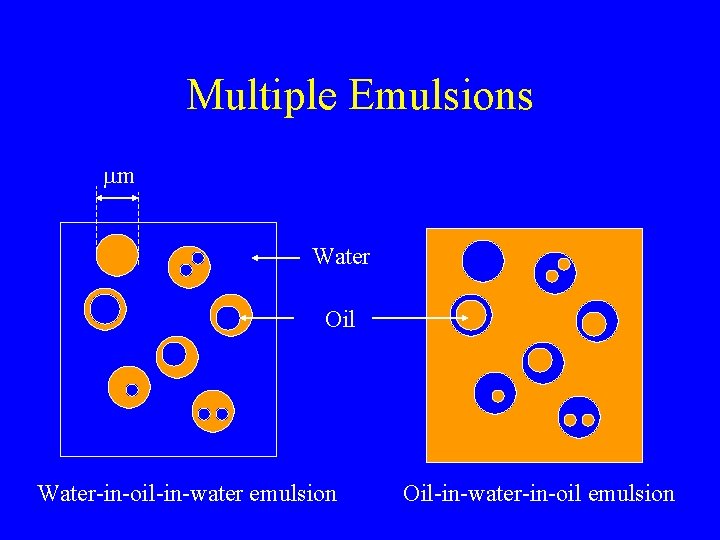
Multiple Emulsions mm Water Oil Water-in-oil-in-water emulsion Oil-in-water-in-oil emulsion

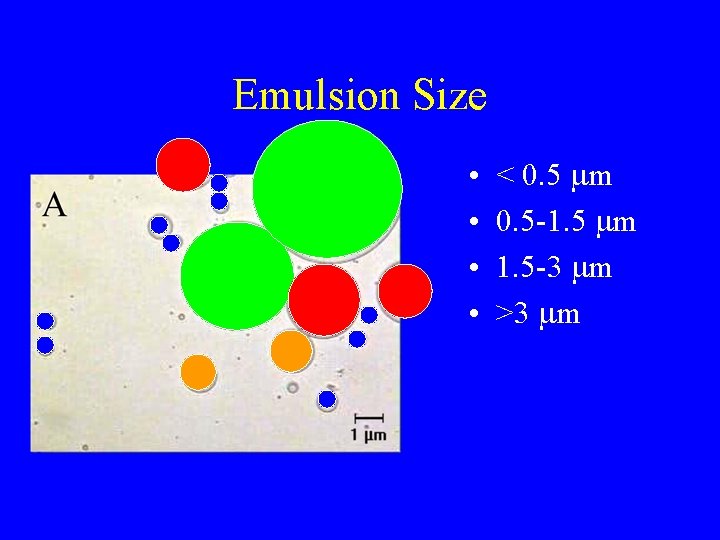
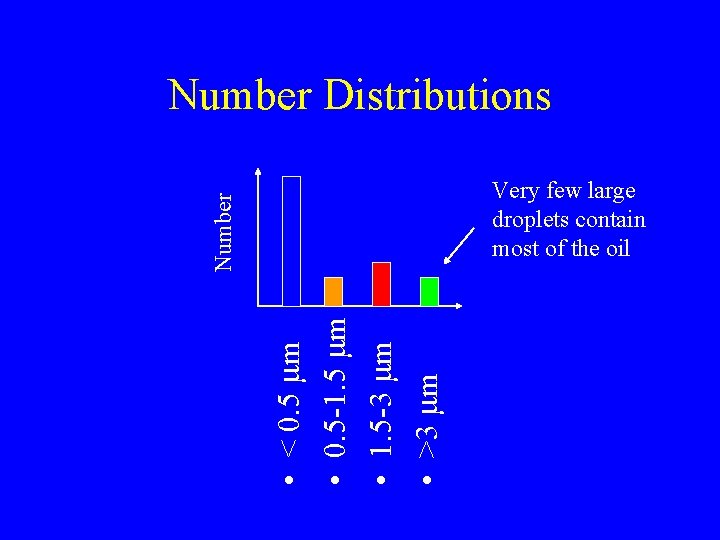
Emulsion Size • • < 0. 5 mm 0. 5 -1. 5 mm 1. 5 -3 mm >3 mm

Number Distributions • • < 0. 5 mm 0. 5 -1. 5 mm 1. 5 -3 mm >3 mm Number Very few large droplets contain most of the oil

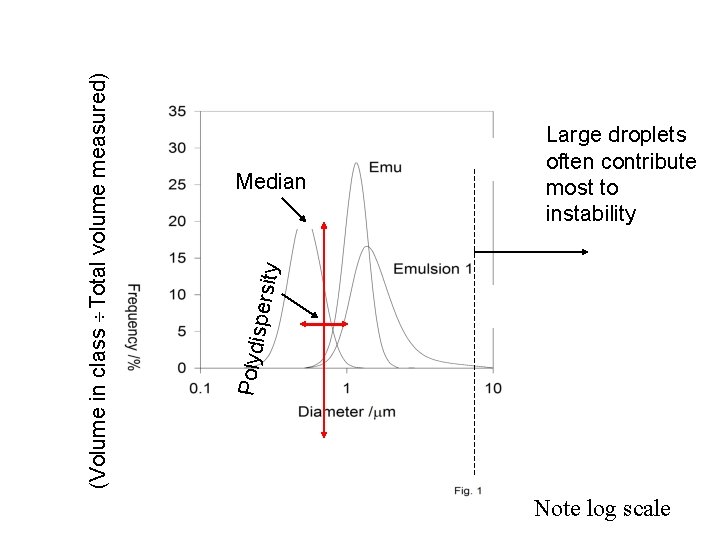
ty spersi Polydi (Volume in class Total volume measured) Median Large droplets often contribute most to instability Note log scale

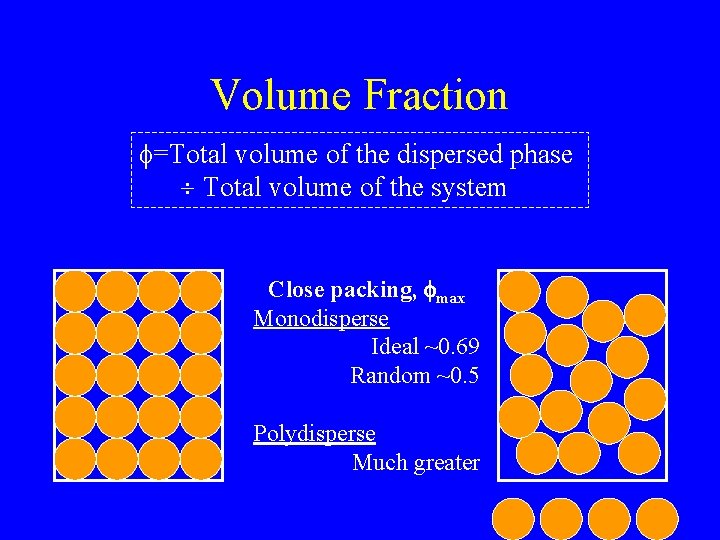
Volume Fraction f=Total volume of the dispersed phase Total volume of the system Close packing, fmax Monodisperse Ideal ~0. 69 Random ~0. 5 Polydisperse Much greater

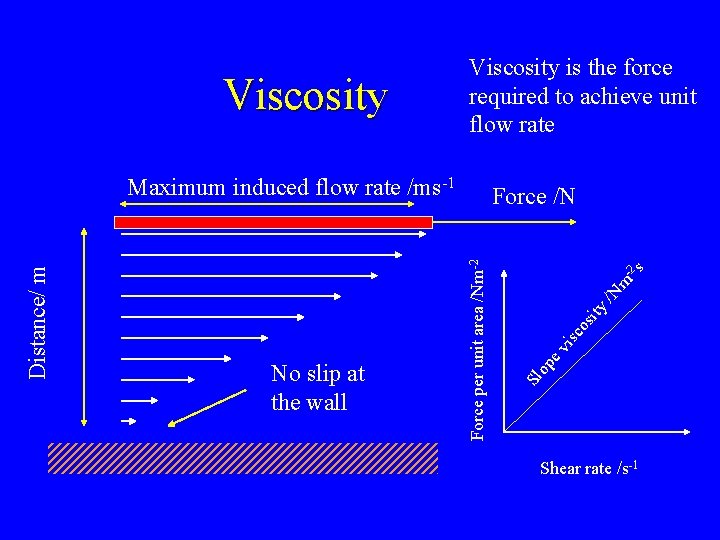
m -2 s /N ity isc os ev op No slip at the wall Force /N Force per unit area /Nm-2 Distance/ m Maximum induced flow rate /ms-1 Sl Viscosity is the force required to achieve unit flow rate Shear rate /s-1

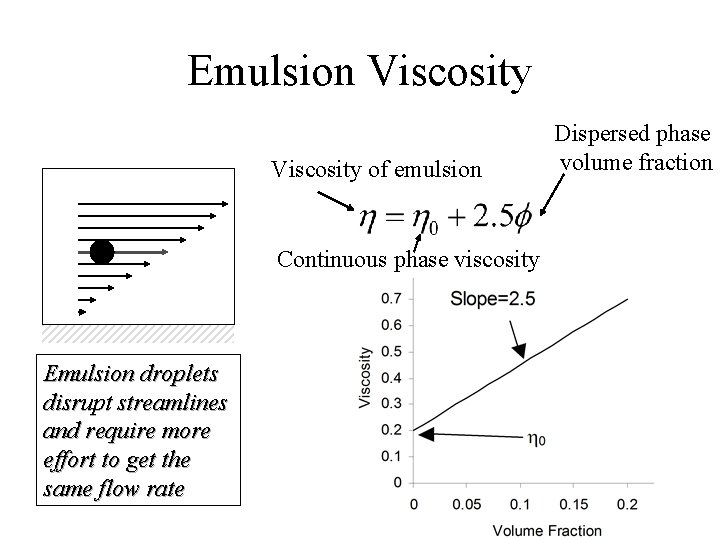
Emulsion Viscosity of emulsion Continuous phase viscosity Emulsion droplets disrupt streamlines and require more effort to get the same flow rate Dispersed phase volume fraction

Chemical Composition Interfacial layer. Essential to stabilizing the emulsion Oil Phase. Limited effects on the properties of the emulsion Aqueous Phase. Aqueous chemical reactions affect the interface and hence emulsion stability

Emulsion Destabilization • • Creaming Flocculation Coalescence Combined methods

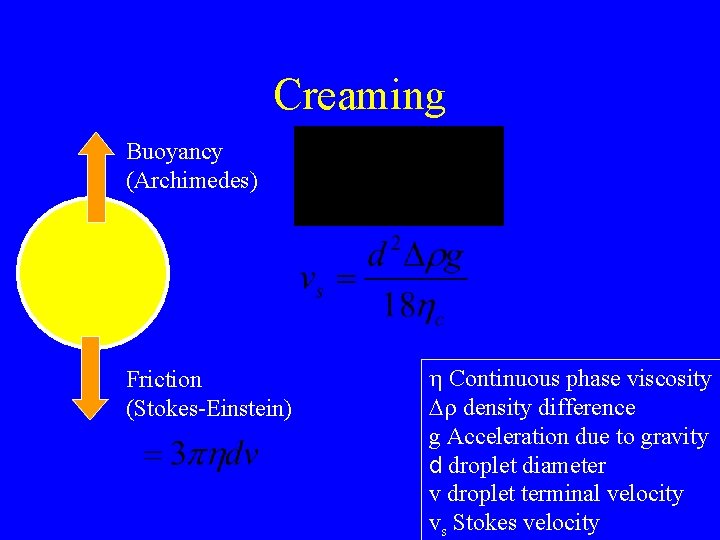
Creaming Buoyancy (Archimedes) Friction (Stokes-Einstein) h Continuous phase viscosity Dr density difference g Acceleration due to gravity d droplet diameter v droplet terminal velocity vs Stokes velocity

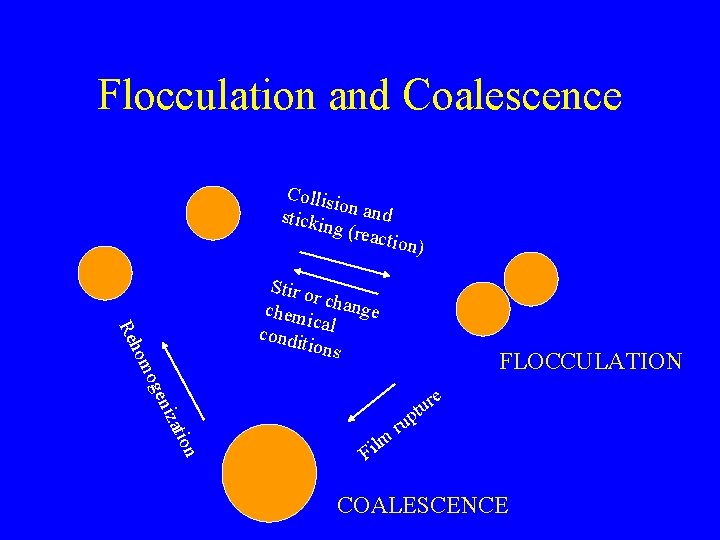
Flocculation and Coalescence Collis io stickin n and g (rea ction) FLOCCULATION atio niz oge hom Re Stir o r chem change ic condi al tions e r u t p n lm i F ru COALESCENCE

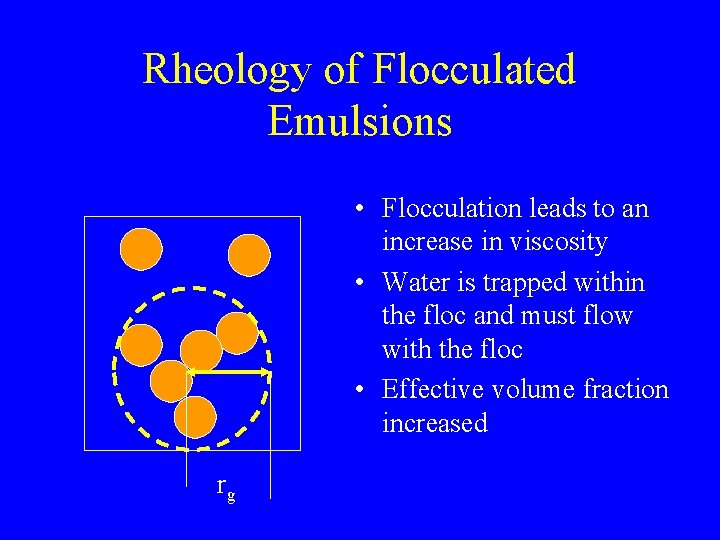
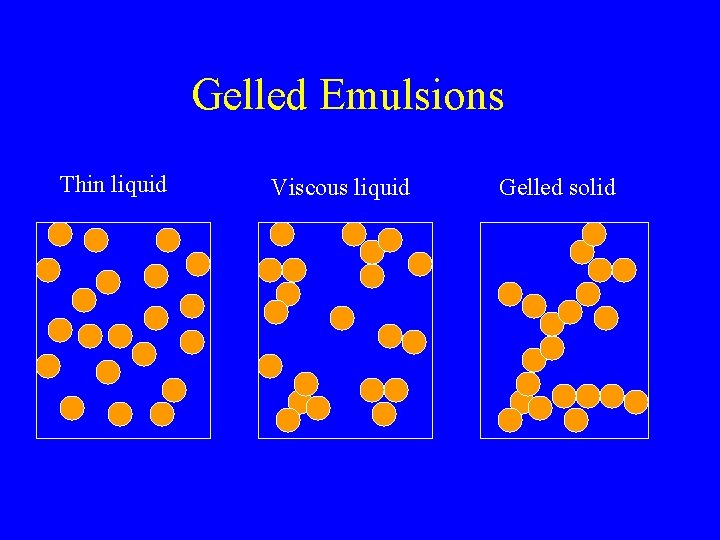
Rheology of Flocculated Emulsions • Flocculation leads to an increase in viscosity • Water is trapped within the floc and must flow with the floc • Effective volume fraction increased rg

Gelled Emulsions Thin liquid Viscous liquid Gelled solid

Creaming & Slight Flocculation • Flocs have larger effective size • Smaller Dr • Tend to cream much faster

Creaming & Extreme Flocculation • Heavily flocculated emulsions form a network • Solid-like properties (gel) • Do not cream (may collapse after lag period)

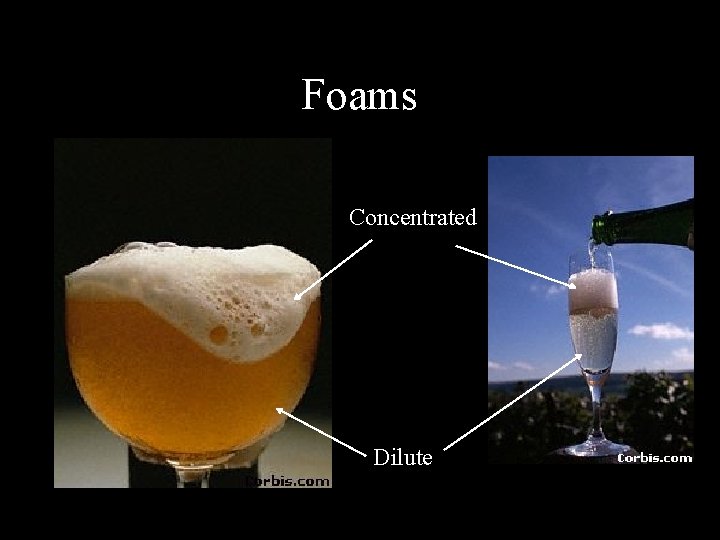
Foams Concentrated Dilute

Dilute Foams • • • Somewhat similar to emulsions Various modes of formation Large (~mm) spherical bubbles Very fast creaming Ostwald ripening

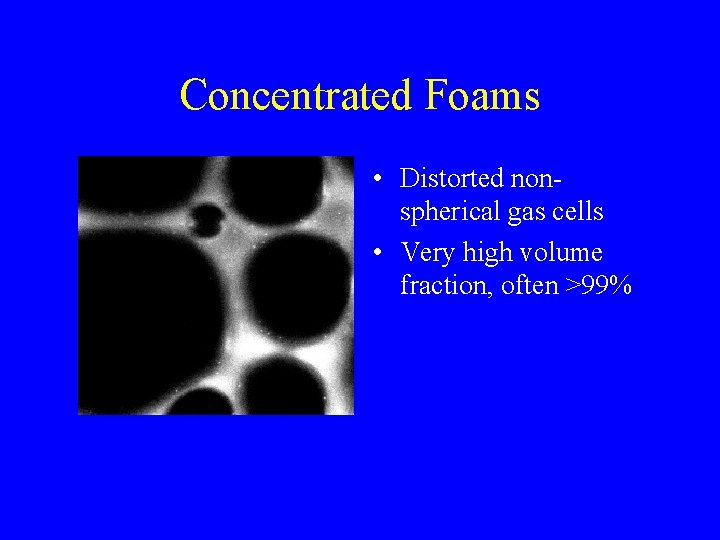
Concentrated Foams • Distorted nonspherical gas cells • Very high volume fraction, often >99%

Foam Drainage • Water drains from foam under gravity • As water leaves, faces of film are brought closer together

Film Rupture • Film must thin then burst • Inhibited by surfactant repulsion/interfacial film • Self-repair by the Gibbs-Marangoni effect
 200+400+600+800
200+400+600+800 These are the richest and sweetest type of baked products
These are the richest and sweetest type of baked products Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Channelstrim
Channelstrim What are the downsides of metal foam
What are the downsides of metal foam Evcl emulsions
Evcl emulsions Auxiliary emulsifying agents examples
Auxiliary emulsifying agents examples Difference between dry gum and wet gum method
Difference between dry gum and wet gum method Identification test for emulsion
Identification test for emulsion Inorganic emulsifying agents examples
Inorganic emulsifying agents examples General goals and specific goals
General goals and specific goals Examples of generic goals and product-specific goals
Examples of generic goals and product-specific goals Intensive property and extensive properties
Intensive property and extensive properties Physical properties and chemical properties
Physical properties and chemical properties What are the positive and negative square roots of 6,400?
What are the positive and negative square roots of 6,400? 63 ün yüzde 60 ı kaçtır
63 ün yüzde 60 ı kaçtır Unlicensed two-way radios
Unlicensed two-way radios Imagine this if you had $86 400
Imagine this if you had $86 400 Sulfametizol blærebetændelse
Sulfametizol blærebetændelse Trotec speedy 100
Trotec speedy 100 Score de sokal
Score de sokal God's silence for 400 years
God's silence for 400 years Average 400 meter time
Average 400 meter time Saresp 2009 uma maquina fotografica
Saresp 2009 uma maquina fotografica