Properties of Elements The Periodic Table Organized by
Properties of Elements
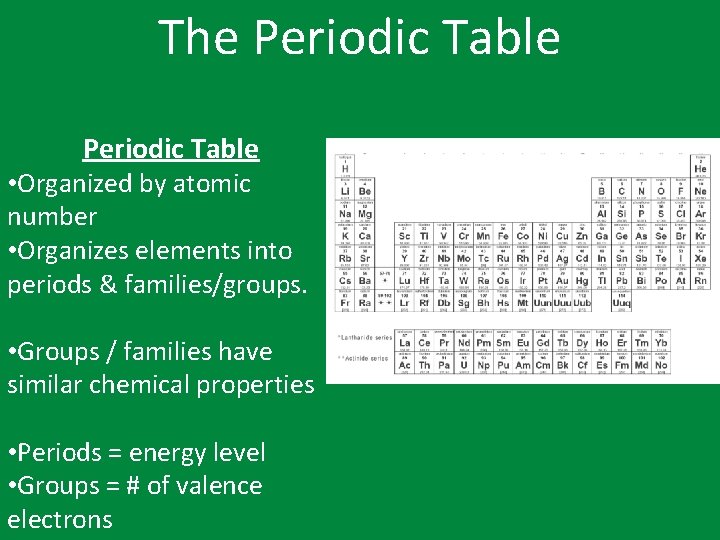
The Periodic Table • Organized by atomic number • Organizes elements into periods & families/groups. • Groups / families have similar chemical properties • Periods = energy level • Groups = # of valence electrons
Metals Physical Properties • Solids at room temp • High density and melting point • Shiny luster • Malleable • Good heat and electrical conductors Chemical Properties • Wide range of reactivities • React w/ non-metals • Tend to lose electrons
Non-Metals Physical Properties • Exist in all 3 states • Low melting points and densities • Brittle • Poor conductors of heat and electricity Chemical Properties • Reactivity varies widely • Tend to gain electrons • React with metals & other n -metals
Metalloids Physical Properties • Physical properties are between those of metals and non-metals. • Some are lustrous • Semi-conductors Chemical Properties • Reactivity depends on other element in compound or reaction • Can gain or lose e-

Alkali Metals Physical Properties • Lower densities than other metals Chemical Properties • React easily, very reactive • Lose 1 outer electron • React readily with the halogens

Alkaline Earth Metals Physical Properties • Physical properties similar to other metals Chemical Properties • React easily • Lose 2 outer electron • React readily with nonmetals
Noble Gases Physical Properties • Gases at room temp • Low Density Chemical Properties • Very unreactive • Stable • 8 (full) outer electrons
Halogens / Halides Physical Properties • Only group with elements in all 3 states of matter Chemical Properties • Very reactive • 7 outer electrons
- Slides: 9