Properties of Elements and Compounds The Periodic Table

Properties of Elements and Compounds
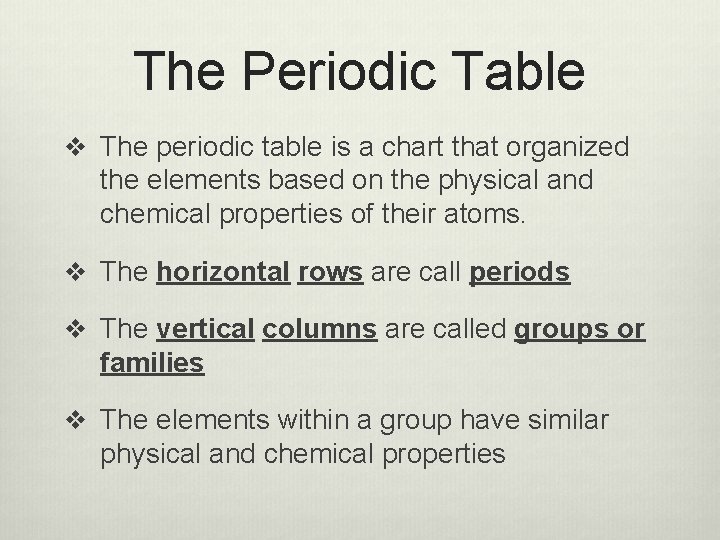
The Periodic Table v The periodic table is a chart that organized the elements based on the physical and chemical properties of their atoms. v The horizontal rows are call periods v The vertical columns are called groups or families v The elements within a group have similar physical and chemical properties
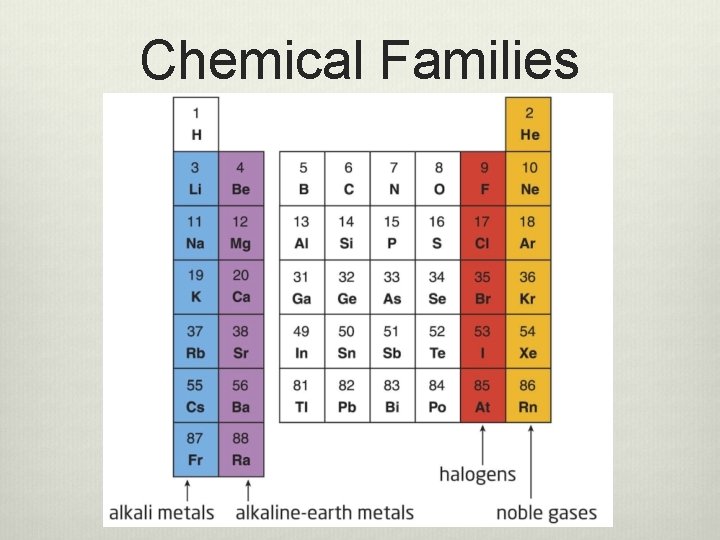
Chemical Families
Chemical Families v Group 1 v Alkali metals v These metals are very v Group 17 v Halogens v Are quite reactive chemically reactive v Group 2 v Alkaline earth metals v These are very reactive, but less reactive than the alkali metals v Group 18 v Noble gases v Almost completely unreactive
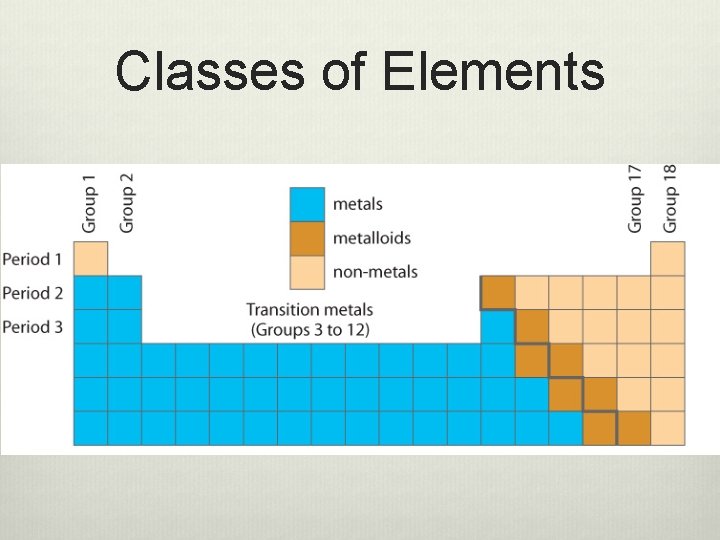
Classes of Elements
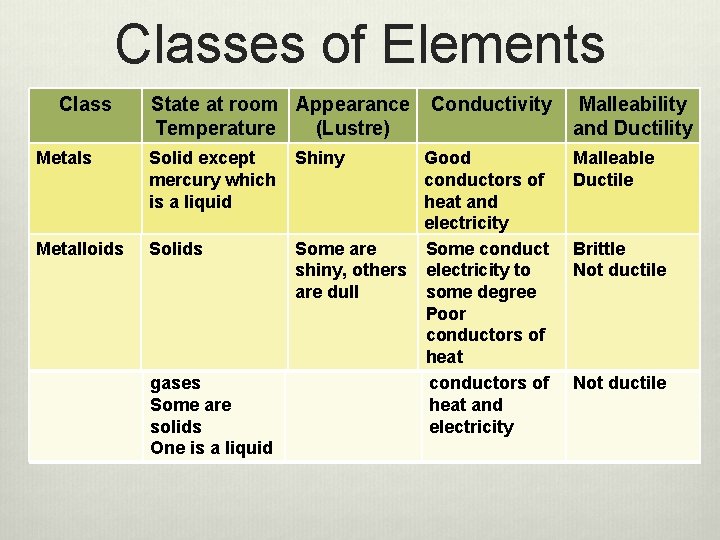
Classes of Elements Class State at room Appearance Temperature (Lustre) Metals Solid except mercury which is a liquid Metalloids Solids Non-metals Some are gases Some are solids One is a liquid Shiny Conductivity Good conductors of heat and electricity Some are Some conduct shiny, others electricity to are dull some degree Poor conductors of heat Not shiny Poor conductors of heat and electricity Malleability and Ductility Malleable Ductile Brittle Not ductile
Bohr Diagrams v Used to show the electrons are arranged v You need to know the number of protons, neutrons and electrons v The number of protons = the number of electrons = the atomic number v To find the number of neutrons: neutrons = atomic mass – atomic number

v Put the number of neutrons and protons in the nucleus (middle) v Put electrons around the nucleus in orbits, also called energy levels v The first orbit can only hold 2 electrons v The second orbit can hold 8 electrons v The third orbit can hold 8 electrons v The fourth orbit can hold 18 electrons
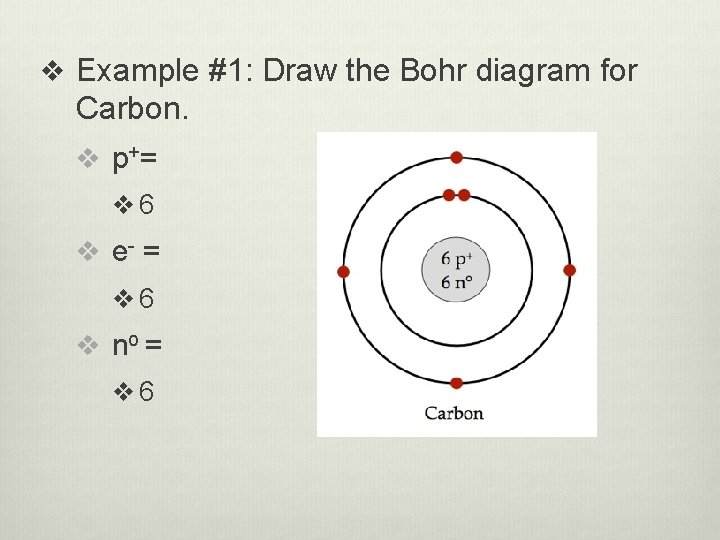
v Example #1: Draw the Bohr diagram for Carbon. v p+ = v 6 v e- = v 6 v no = v 6
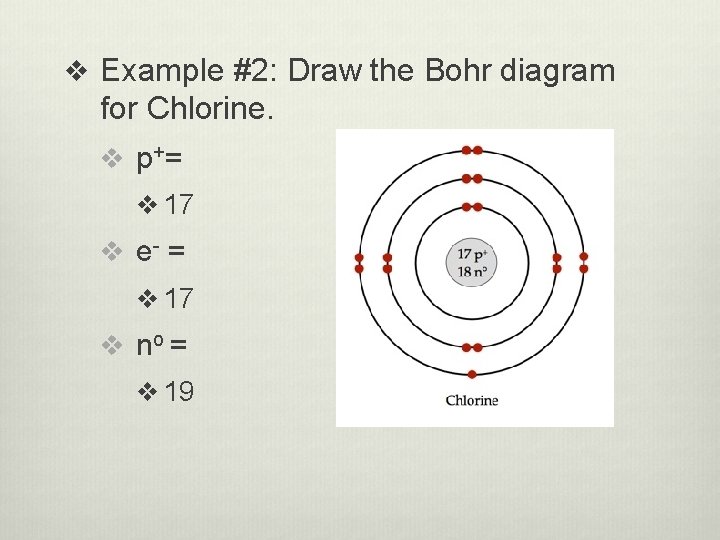
v Example #2: Draw the Bohr diagram for Chlorine. v p+ = v 17 v e- = v 17 v no = v 19
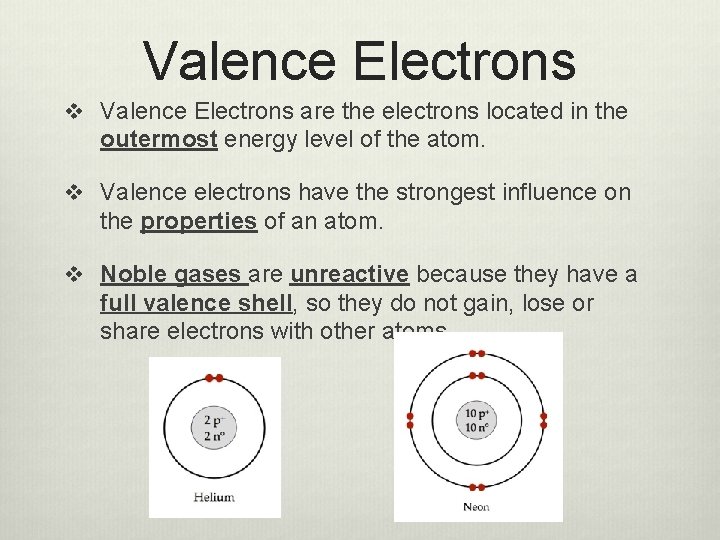
Valence Electrons v Valence Electrons are the electrons located in the outermost energy level of the atom. v Valence electrons have the strongest influence on the properties of an atom. v Noble gases are unreactive because they have a full valence shell, so they do not gain, lose or share electrons with other atoms.
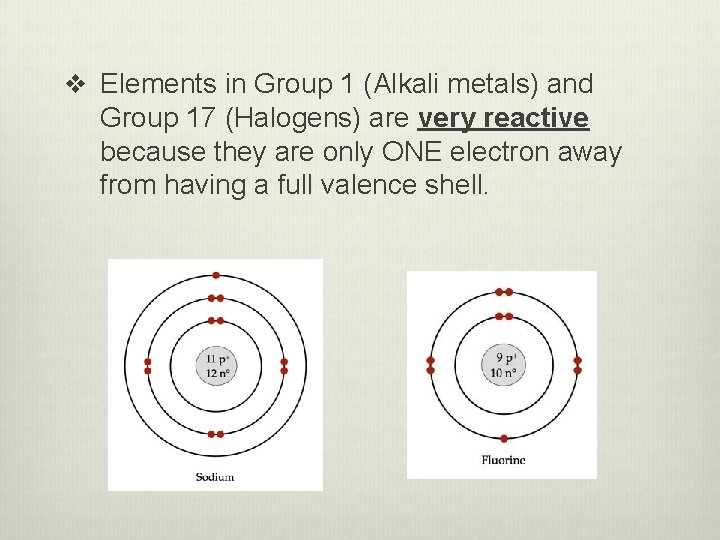
v Elements in Group 1 (Alkali metals) and Group 17 (Halogens) are very reactive because they are only ONE electron away from having a full valence shell.

Practice v Text Book: v Page 3 # 7 v Page 4 # 9 and 10
- Slides: 13