Properties of Cytokines Abbas Chpt 11 Fig 11

Properties of Cytokines Abbas: Chpt. 11; Fig. 11. 2
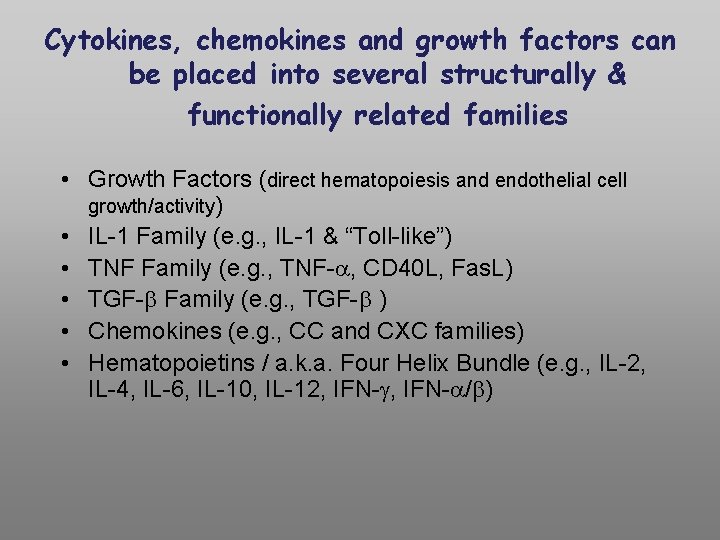
Cytokines, chemokines and growth factors can be placed into several structurally & functionally related families • Growth Factors (direct hematopoiesis and endothelial cell growth/activity) • IL-1 Family (e. g. , IL-1 & “Toll-like”) • TNF Family (e. g. , TNF- , CD 40 L, Fas. L) • TGF- Family (e. g. , TGF- ) • Chemokines (e. g. , CC and CXC families) • Hematopoietins / a. k. a. Four Helix Bundle (e. g. , IL-2, IL-4, IL-6, IL-10, IL-12, IFN- / )

Figure 2 -39
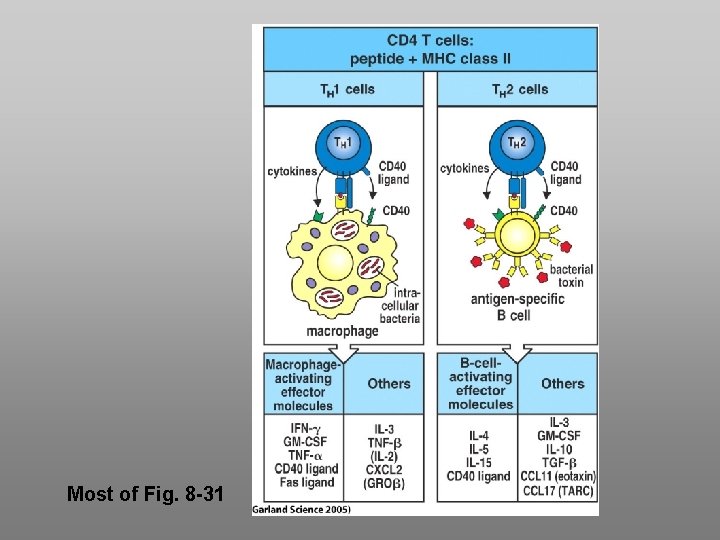
Most of Fig. 8 -31
Let’s digress to review TCR signaling for an important clinical pearl!
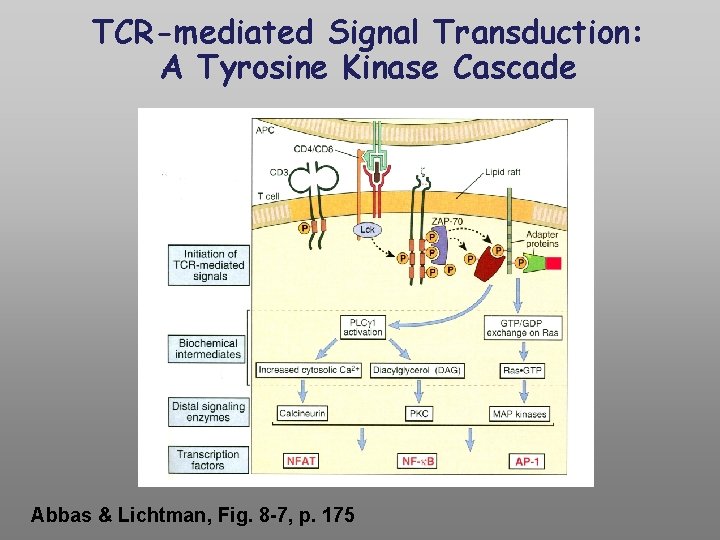
TCR-mediated Signal Transduction: A Tyrosine Kinase Cascade Abbas & Lichtman, Fig. 8 -7, p. 175
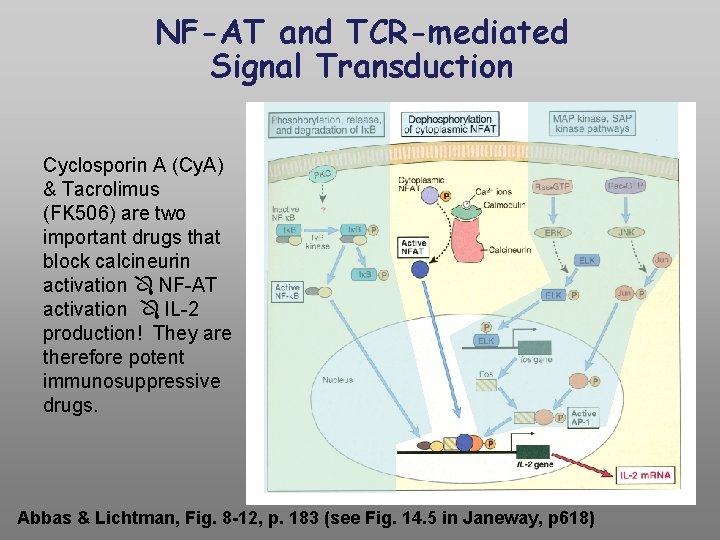
NF-AT and TCR-mediated Signal Transduction Cyclosporin A (Cy. A) & Tacrolimus (FK 506) are two important drugs that block calcineurin activation NF-AT activation IL-2 production! They are therefore potent immunosuppressive drugs. Abbas & Lichtman, Fig. 8 -12, p. 183 (see Fig. 14. 5 in Janeway, p 618)
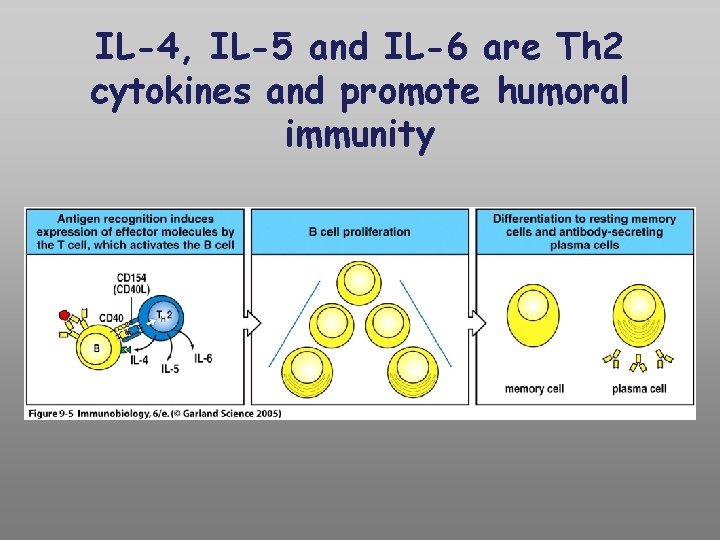
IL-4, IL-5 and IL-6 are Th 2 cytokines and promote humoral immunity
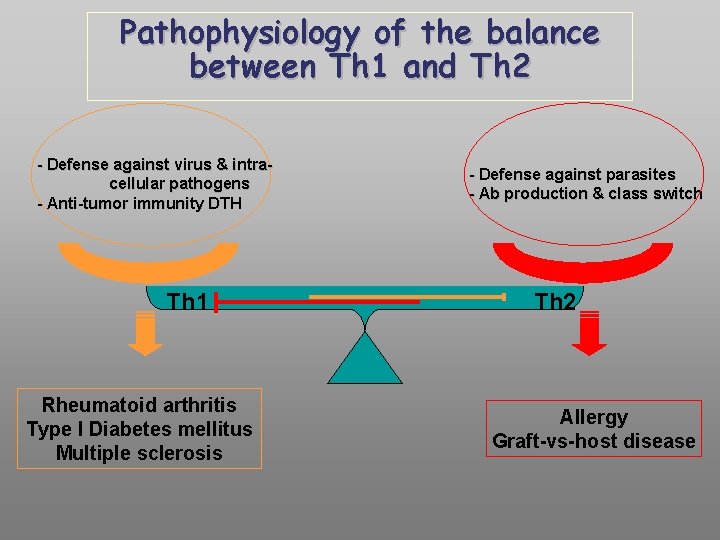
Pathophysiology of the balance between Th 1 and Th 2 - Defense against virus & intracellular pathogens - Anti-tumor immunity DTH Th 1 Rheumatoid arthritis Type I Diabetes mellitus Multiple sclerosis - Defense against parasites - Ab production & class switch Th 2 Allergy Graft-vs-host disease

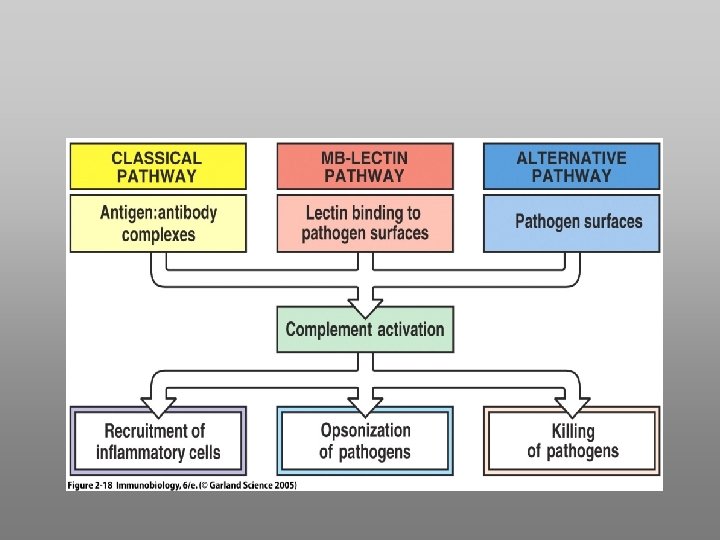
Functions of Complement A. Host Defense B. Disposal of Waste C. Regulation of the Immune Response

Functions of Complement Disposal of Waste Immune Complex Removal Apoptotic Cell Debris Removal

Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial Pathogens
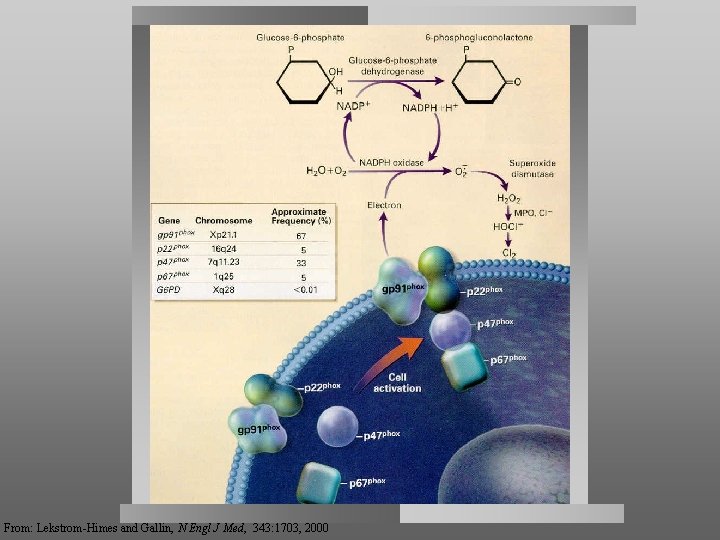
From: Lekstrom-Himes and Gallin, N Engl J Med, 343: 1703, 2000
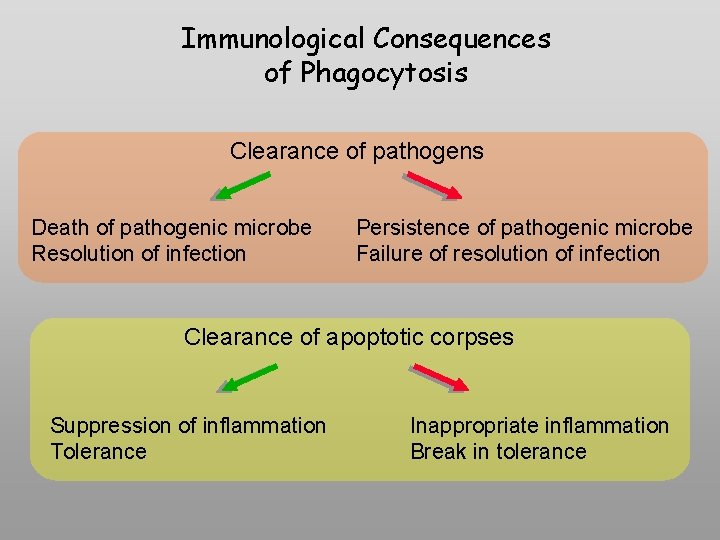
Immunological Consequences of Phagocytosis Clearance of pathogens Death of pathogenic microbe Resolution of infection Persistence of pathogenic microbe Failure of resolution of infection Clearance of apoptotic corpses Suppression of inflammation Tolerance Inappropriate inflammation Break in tolerance
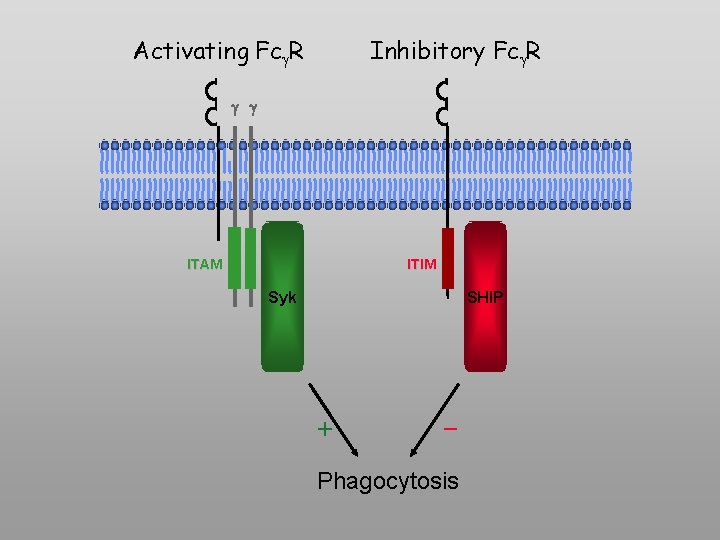
Activating Fc R Inhibitory Fc R g g ITAM ITIM Syk SHIP + - Phagocytosis
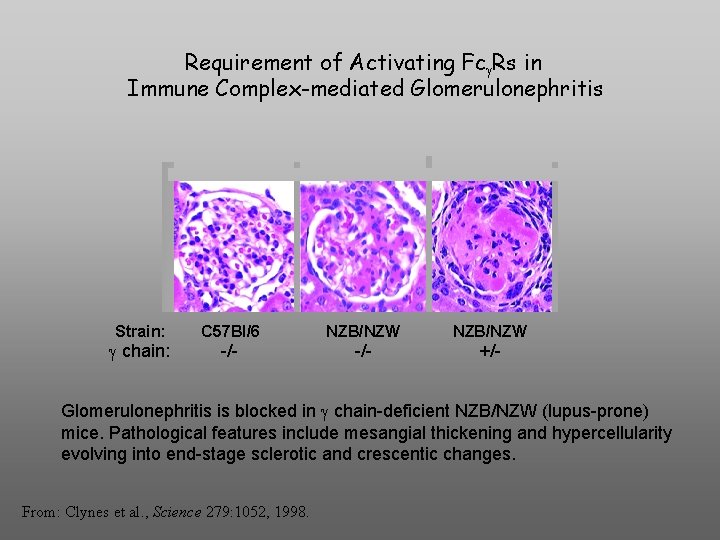
Requirement of Activating Fc Rs in Immune Complex-mediated Glomerulonephritis Strain: chain: C 57 Bl/6 NZB/NZW -/- +/- Glomerulonephritis is blocked in chain-deficient NZB/NZW (lupus-prone) mice. Pathological features include mesangial thickening and hypercellularity evolving into end-stage sclerotic and crescentic changes. From: Clynes et al. , Science 279: 1052, 1998.

Summary 1. 2. 3. Phagocytosis is a component of innate and aquired immunity. It is the principal means of destroying pathogenic bacteria and fungi. Phagocytosis initiates the process of antigen presentation. 2. 3. Many phagocytic receptors recognize a diverse array of microbial pathogens. Some pathogens (e. g. , S. pneumoniae) require opsonization for their clearance. 3. Bugs fight back. 4. 5. 6. Phagocytosis is an essential component of development and tissue remodeling. Ingestion of apoptotic bodies is immunologically “silent” and is normally accompanied by a suppression of inflammation. 5. Failure of this mechanism may result in autoimmunity. 6. 7. Fc receptors come in two basic types: activating (ITAM-associated) and inhibitory (ITIM-associated). 7. 8. The relative expression of activating and inhibitory Fc receptors determines the outcome of a given engagement of Fc receptors. 8. 9. Fc receptor-driven pathology includes formation and deposition of immune complexes, which play a major role in autoimmunity.

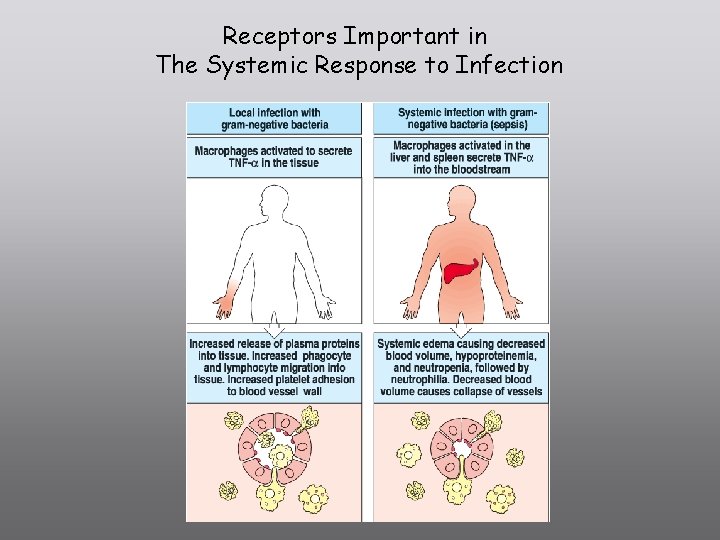
Receptors Important in The Systemic Response to Infection
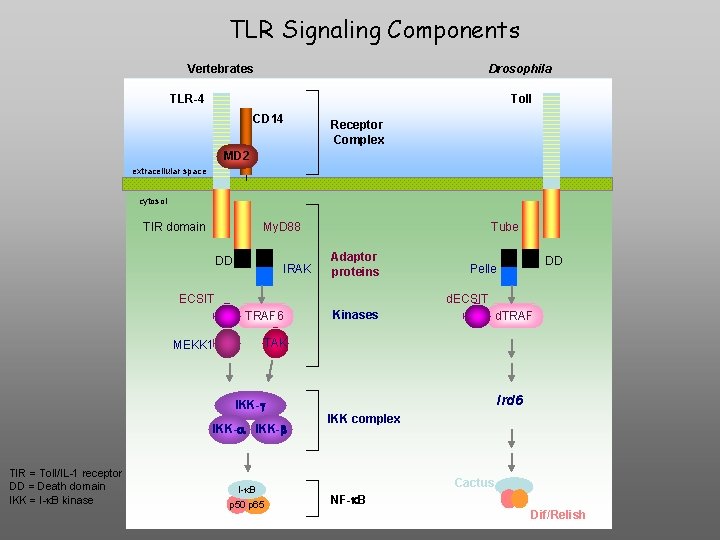
TLR Signaling Components Vertebrates Drosophila TLR-4 Toll CD 14 Receptor Complex MD 2 extracellular space cytosol TIR domain My. D 88 DD IRAK Tube Adaptor proteins ECSIT d. ECSIT TRAF 6 MEKK 1 Kinases d. TRAF TAK Ird 6 IKK-g IKK-a IKK-b TIR = Toll/IL-1 receptor DD = Death domain IKK = I-k. B kinase DD Pelle I-k. B p 50 p 65 IKK complex Cactus NF-k. B Dif/Relish
The (Primary) Acquired Immune Response is Initiated by Innate Immune Recognition
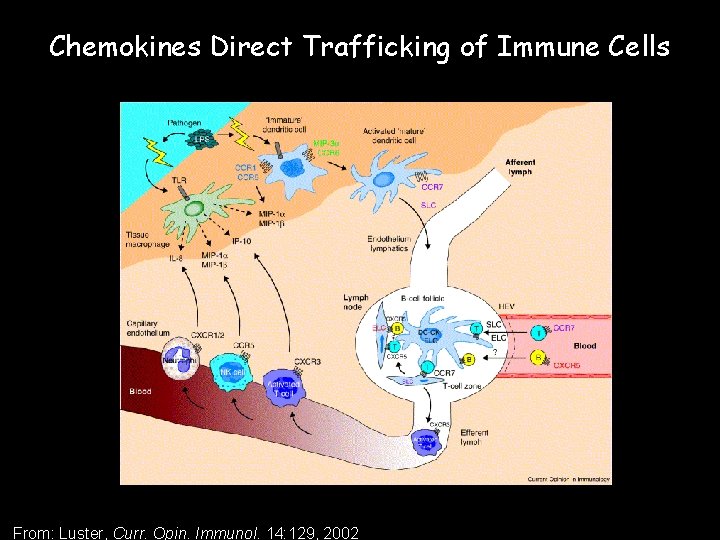
Chemokines Direct Trafficking of Immune Cells From: Luster, Curr. Opin. Immunol. 14: 129, 2002
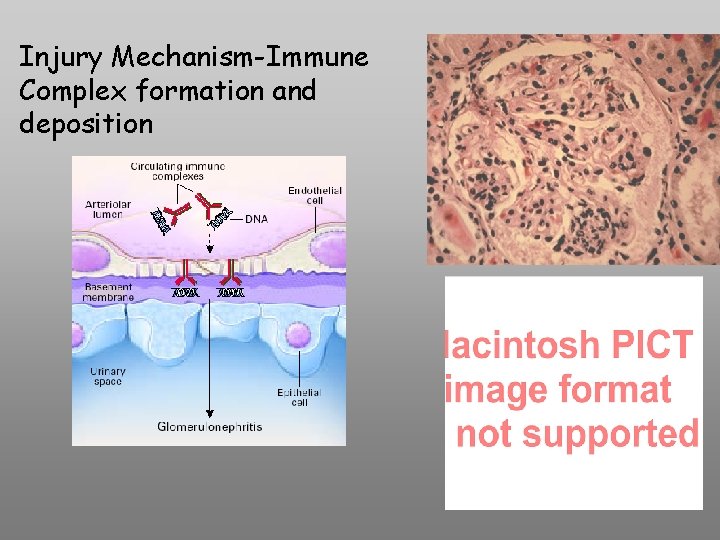
Injury Mechanism-Immune Complex formation and deposition
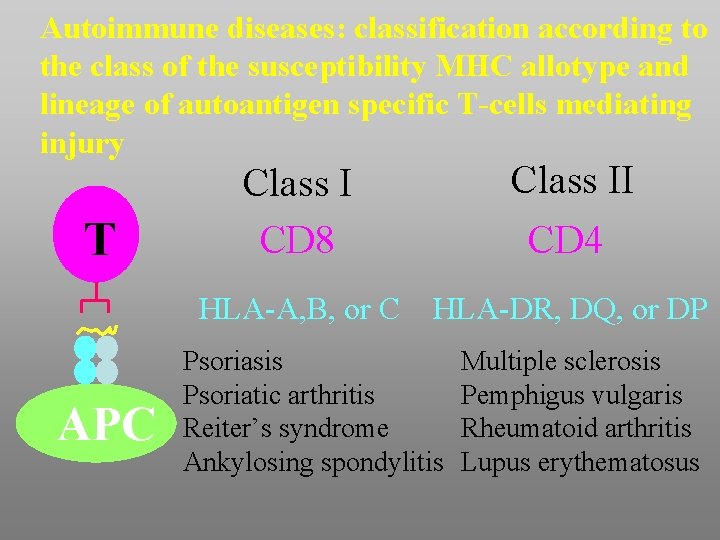
Autoimmune diseases: classification according to the class of the susceptibility MHC allotype and lineage of autoantigen specific T-cells mediating injury T APC Class I CD 8 Class II CD 4 HLA-A, B, or C HLA-DR, DQ, or DP Psoriasis Psoriatic arthritis Reiter’s syndrome Ankylosing spondylitis Multiple sclerosis Pemphigus vulgaris Rheumatoid arthritis Lupus erythematosus
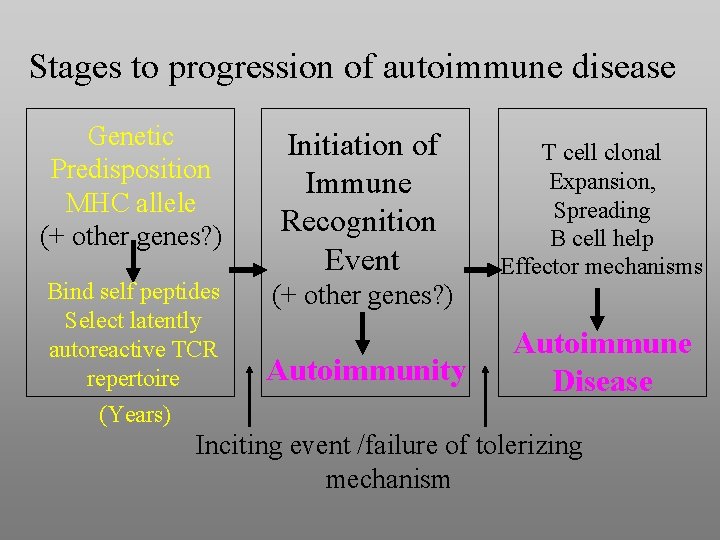
Stages to progression of autoimmune disease Genetic Predisposition MHC allele (+ other genes? ) Initiation of Immune Recognition Event Bind self peptides Select latently autoreactive TCR repertoire (Years) (+ other genes? ) Autoimmunity T cell clonal Expansion, Spreading B cell help Effector mechanisms Autoimmune Disease Inciting event /failure of tolerizing mechanism
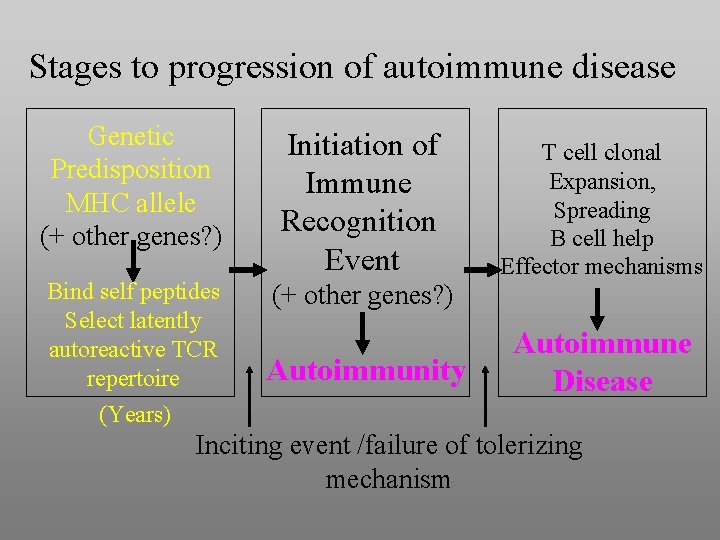
Stages to progression of autoimmune disease Genetic Predisposition MHC allele (+ other genes? ) Initiation of Immune Recognition Event Bind self peptides Select latently autoreactive TCR repertoire (Years) (+ other genes? ) Autoimmunity T cell clonal Expansion, Spreading B cell help Effector mechanisms Autoimmune Disease Inciting event /failure of tolerizing mechanism
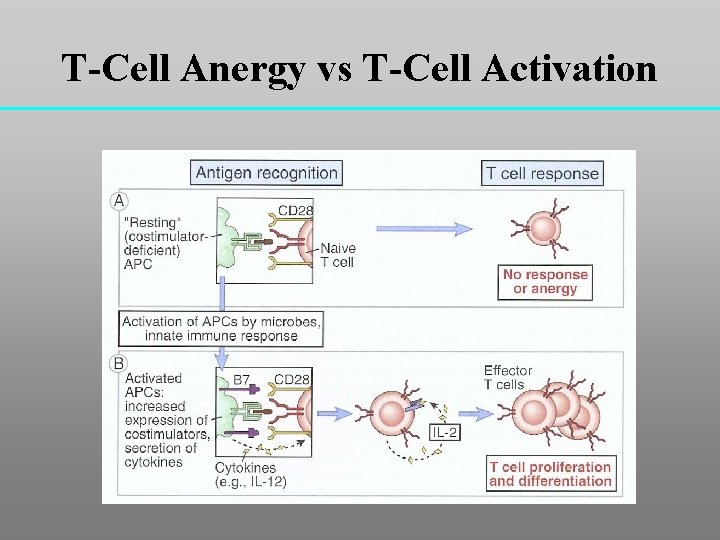
T-Cell Anergy vs T-Cell Activation
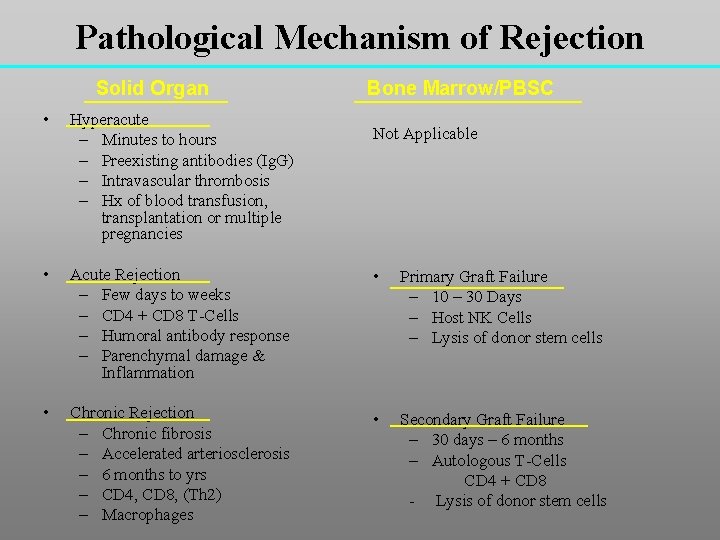
Pathological Mechanism of Rejection Solid Organ Bone Marrow/PBSC • Hyperacute – Minutes to hours – Preexisting antibodies (Ig. G) – Intravascular thrombosis – Hx of blood transfusion, transplantation or multiple pregnancies • Acute Rejection – Few days to weeks – CD 4 + CD 8 T-Cells – Humoral antibody response – Parenchymal damage & Inflammation • Primary Graft Failure – 10 – 30 Days – Host NK Cells – Lysis of donor stem cells • Chronic Rejection – Chronic fibrosis – Accelerated arteriosclerosis – 6 months to yrs – CD 4, CD 8, (Th 2) – Macrophages • Secondary Graft Failure – 30 days – 6 months – Autologous T-Cells CD 4 + CD 8 - Lysis of donor stem cells Not Applicable
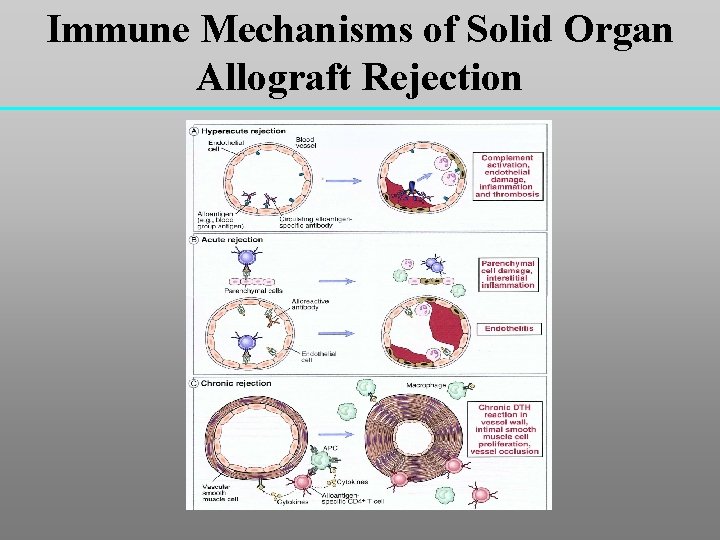
Immune Mechanisms of Solid Organ Allograft Rejection
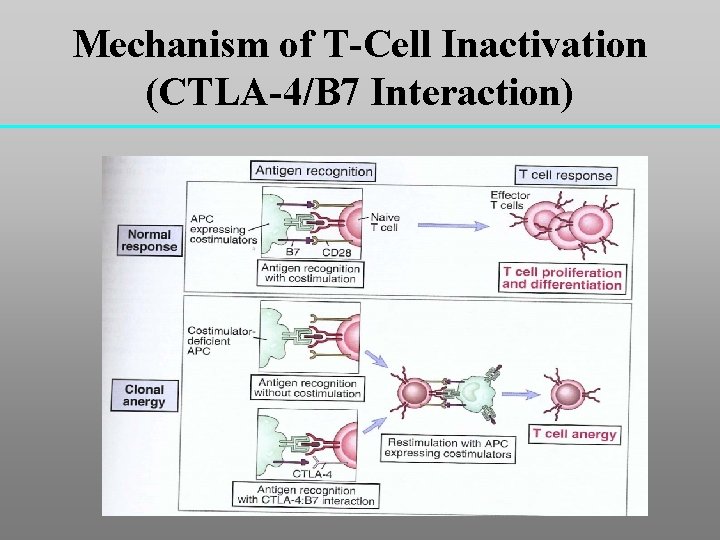
Mechanism of T-Cell Inactivation (CTLA-4/B 7 Interaction)

ORAL TOLERANCE • ORAL ADMINISTRATION OF A PROTEIN ANTIGEN MAY LEAD TO SUPPRESSION OF SYSTEMIC HUMORAL AND CELL-MEDIATED IMMUNE RESPONSES TO IMMUNIZATION WITH THE SAME ANTIGEN. • POSSIBLE MECHANISMS: – INDUCTION OF ANERGY OF ANTIGEN-SPECIFIC T CELLS – CLONAL DELETION OF ANTIGEN-SPECIFIC T CELLS – SELECTIVE EXPANSION OF CELLS PRODUCING IMMUNOSUPPRESSIVE CYTOKINES (IL-4, IL-10, TGF-b)

REGULATORY T CELLS (CD 4+) • TH 3 CELLS: A POPULATION OF CD 4+T CELLS THAT PRODUCE TGF-. ISOLATED FROM MICE FED LOW DOSE OF ANTIGEN FOR TOLERANCE INDUCTION • TR 1 CELLS: A POPULATION OF CD 4+T CELLS THAT PRODUCE IL-10. CAN PRODUCE SUPPRESSION OF EXPERIMENTAL COLITIS IN MICE • CD 4+CD 25+ REGULATORY T CELLS: A POPULATION OF CD 4+T CELLS THAT CAN PREVENT AUTOREACTIVITY IN VIVO.
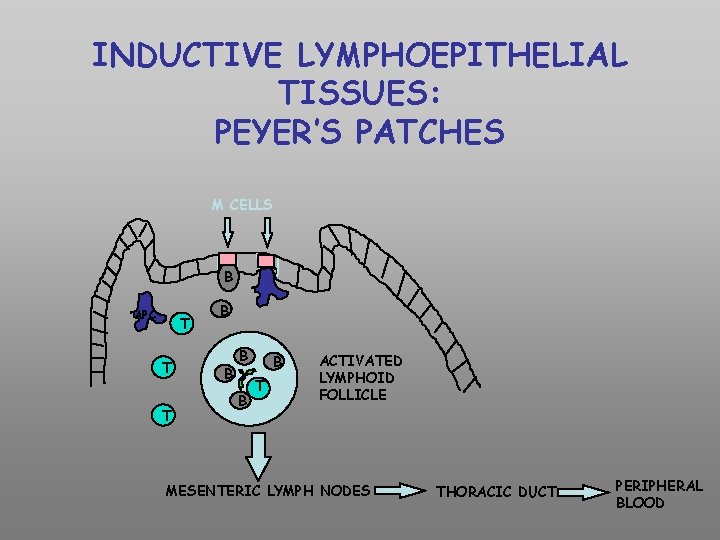
INDUCTIVE LYMPHOEPITHELIAL TISSUES: PEYER’S PATCHES M CELLS B APC T T T B B B T ACTIVATED LYMPHOID FOLLICLE MESENTERIC LYMPH NODES THORACIC DUCT PERIPHERAL BLOOD
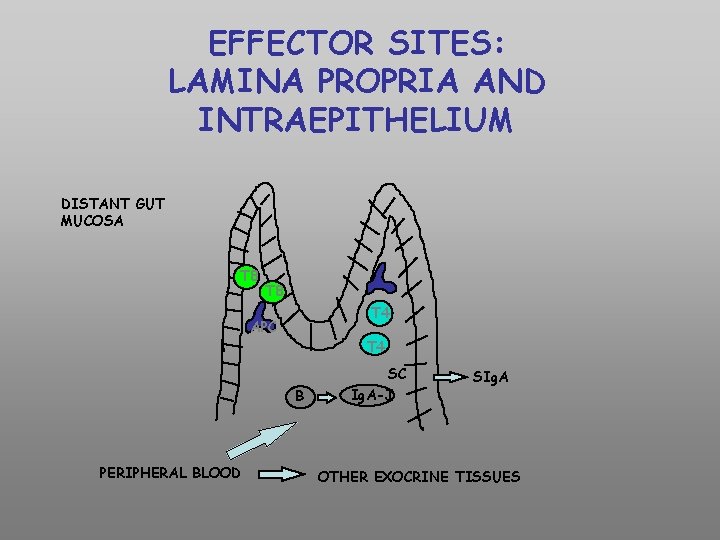
EFFECTOR SITES: LAMINA PROPRIA AND INTRAEPITHELIUM DISTANT GUT MUCOSA T 8 T 4 APC T 4 B PERIPHERAL BLOOD SC Ig. A-J SIg. A OTHER EXOCRINE TISSUES

Inflammatory Bowel Disease: Immunological Features • HUMORAL IMMUNITY: MASSIVE INCREASE IN THE NUMBER OF PLASMA CELLS AND IN Ig. G PRODUCTION (Ig. G 2 IN CD AND Ig. G 1 IN UC) • IMBALANCE OF PRO-INFLAMMATORY (TNF-a, IL-1, IL-8, IL-12) AND ANTI-INFLAMMATORY CYTOKINES (IL-10, IL-4, IL-13)
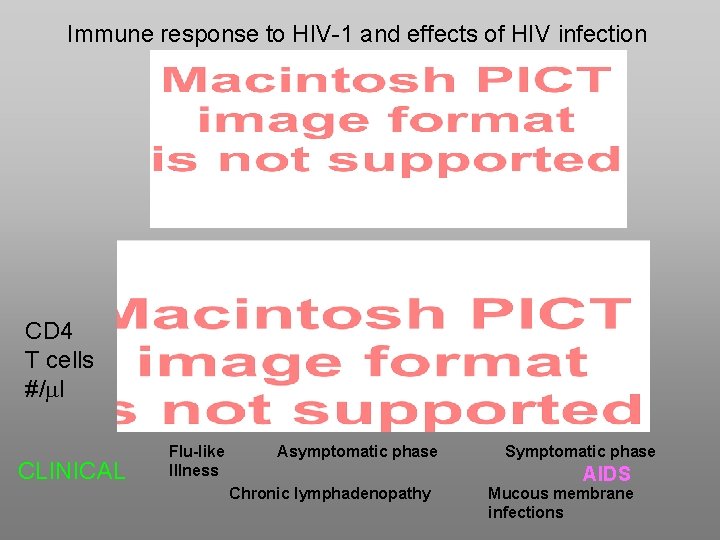
Immune response to HIV-1 and effects of HIV infection CD 4 T cells #/ml CLINICAL Flu-like Illness Asymptomatic phase Chronic lymphadenopathy Symptomatic phase AIDS Mucous membrane infections

HIV strain early in infection • R 5 is almost always the sexually transmissible form of the virus • Primary isolates from newly infected individuals are usually R 5 • R 5 strains mainly replicate in monocytes. Activated and memory T cells are infected, but at lower efficiency (old term = MT-tropic or monocytotropic) • Therefore much of the viral load in earlier phase of HIV infection is in the monocytes and macrophages and the numbers of CD 4 T cells remains stable, but decreased
CD 8 T-cell Response to HIV-1 • Establishes asymptomatic phase of infection • The CD 8 T-cell responds to HIV-peptides by activation, clonal expansion, and differentiation to effector status • Specific lysis of HIV- infected target cells (macrophages and CD 4 T cells) via perforin pathway and/ or apoptosis via upregulation of fas ligand • Strong inhibition of viral infectivity by release of chemokines (MIP-1 / , RANTES) that bind to CCR 5 and block coreceptor dependent entry of R 5 HIV-1 • Release of IFN- and secondarily TNF- , decrease LTR-driven transcription

Thwarted immunosurveillance (2) Dendritic cells used as a “Trojan Horse” • Immature DCs, typically located in the submucosa express a C-type lectin DC-SIGN • HIV-1 envelope binds to DC-SIGN with high affinity • The virions are internalized and remain in acidic endosomal compartments while the DC matures • Intact infectious virions are reexpressed on the surface when the DC enters the lymph node

Viral Response near end of asymptomatic period • Rate of viral infection and potential mutations increases. Definitive viral escape occurs when virus is no longer presented by MHC to available CD 8 T cell clones • Continual generation of env mutations • Selection against R 5 variants by CD 8 T-cell CCR 5 chemokines that blocks infection is finally bypassed • Change in cellular tropism by env mutations leads to X 4 phenotype (CXCR 4, Ttropic) • Enhanced T-tropism of X 4 leads to more significant impairment of CD 4 T-cell compartment Loss of the “epitope war”
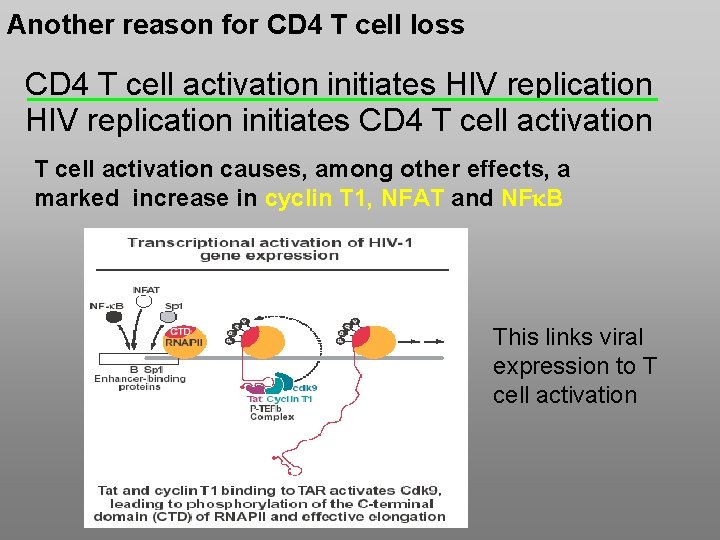
Another reason for CD 4 T cell loss CD 4 T cell activation initiates HIV replication initiates CD 4 T cell activation causes, among other effects, a marked increase in cyclin T 1, NFAT and NFk. B This links viral expression to T cell activation
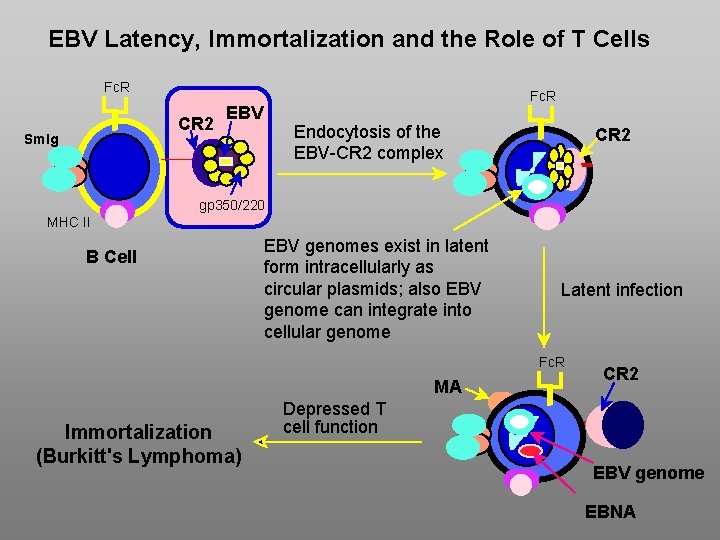
EBV Latency, Immortalization and the Role of T Cells Fc. R CR 2 Sm. Ig Fc. R EBV Endocytosis of the EBV-CR 2 complex CR 2 gp 350/220 MHC II B Cell EBV genomes exist in latent form intracellularly as circular plasmids; also EBV genome can integrate into cellular genome Latent infection Fc. R MA Immortalization (Burkitt's Lymphoma) CR 2 Depressed T cell function EBV genome EBNA
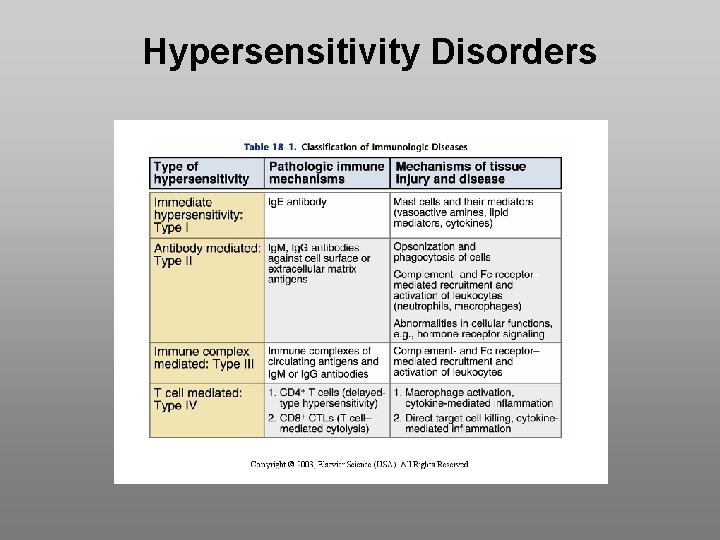
Hypersensitivity Disorders
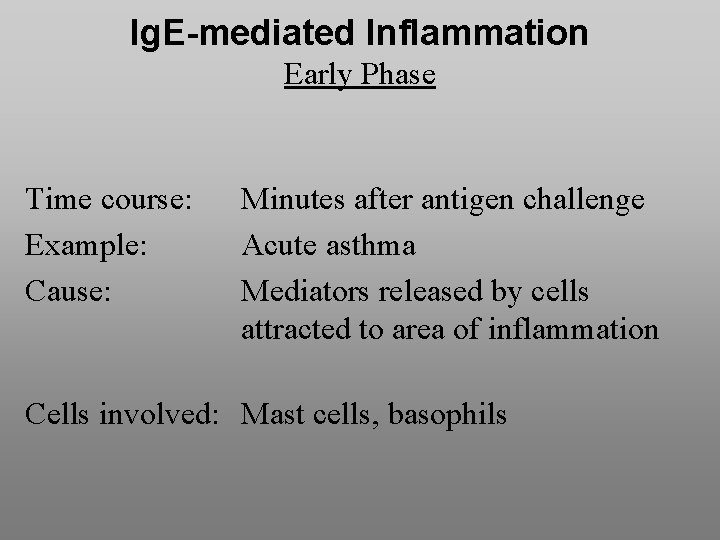
Ig. E-mediated Inflammation Early Phase Time course: Example: Cause: Minutes after antigen challenge Acute asthma Mediators released by cells attracted to area of inflammation Cells involved: Mast cells, basophils
Ig. E-mediated Inflammation Late Phase Time course: Example: Cause: Hours after antigen challenge Chronic asthma Mediators released by cells attracted to area of inflammation during and after the early phase Cells involved: Eosinophils, Basophils Neutrophils, Lymphocytes
Control of Ig. E Production (Candidate Genes) I. Localization to specific chromosomes a. Chromosome 5 q - Promoter variants for IL-4 (IL-3, -5, -9, -13 and GM-CSF) b. Chromosome 11 q Subunit of Fc RI (High affinity Ig. E receptor) c. Others II. HLA linkage to specific antigen responses

“Hygiene Hypothesis” • Observation (one of a number of examples) – Children raised in rural areas close to animals and exposed to endotoxin in dust have a lower incidence of atopic disease • Theory – Endotoxin acting on Toll-like receptors influences the cytokines that APC’s secrete as they present antigen so as to favor a Th 1 instead of a Th 2 response
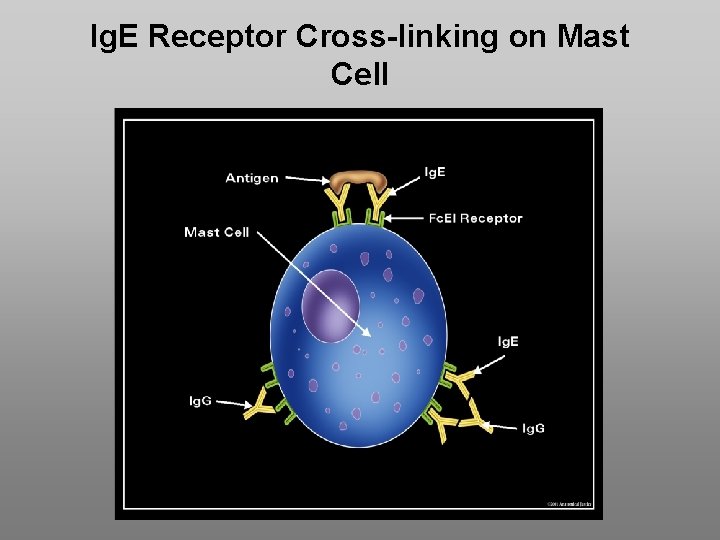
Ig. E Receptor Cross-linking on Mast Cell
Inflammatory Mediators Mast Cells and Basophils Histamine Leukotrienes C 4, D 4, E 4 Platelet Activating Factor (PAF) TNF- , IL-4, IL-13 Mast Cells Only PGD 2 Tryptase (Used to detect anaphylaxis) IL-5, -6
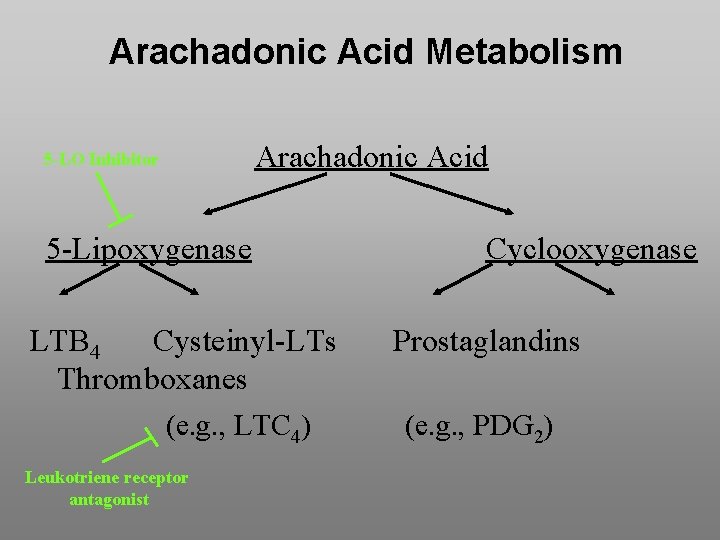
Arachadonic Acid Metabolism Arachadonic Acid 5 -LO Inhibitor 5 -Lipoxygenase LTB 4 Cysteinyl-LTs Thromboxanes (e. g. , LTC 4) Leukotriene receptor antagonist Cyclooxygenase Prostaglandins (e. g. , PDG 2)
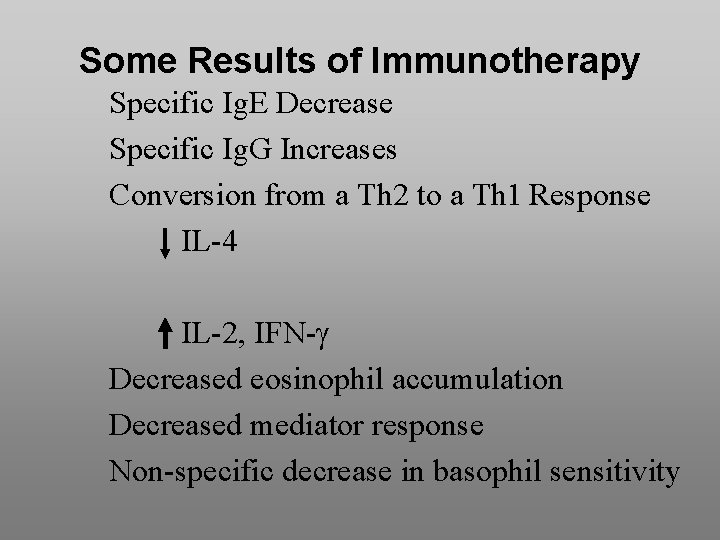
Some Results of Immunotherapy Specific Ig. E Decrease Specific Ig. G Increases Conversion from a Th 2 to a Th 1 Response IL-4 IL-2, IFN- Decreased eosinophil accumulation Decreased mediator response Non-specific decrease in basophil sensitivity
- Slides: 52