Properties of Covalent Molecules Learning Objectives 1 Describe
Properties of Covalent Molecules Learning Objectives: 1. Describe the properties of simple covalent molecules including melting and boiling point, physical state, and electrical conductivity. 2. Describe the properties of polymers. 3. Describe the properties of giant covalent structures. 4. Explain the properties of covalent molecules using ideas about intermolecular forces and electrical charge. 5. Compare the structures and properties of different forms of carbon: a) Diamond b) Graphite c) Graphene and fullerenes
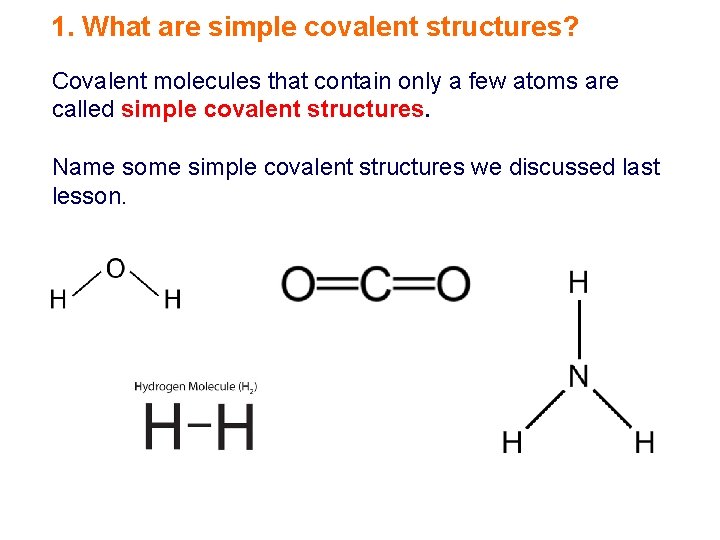
1. What are simple covalent structures? Covalent molecules that contain only a few atoms are called simple covalent structures. Name some simple covalent structures we discussed last lesson.
2. Properties of Simple Covalent Molecules • Watch the demonstration and write down the properties of simple covalent molecules. Including: a) Physical State (at room temperature) b) Melting and Boiling Point c) Electrical Conductivity
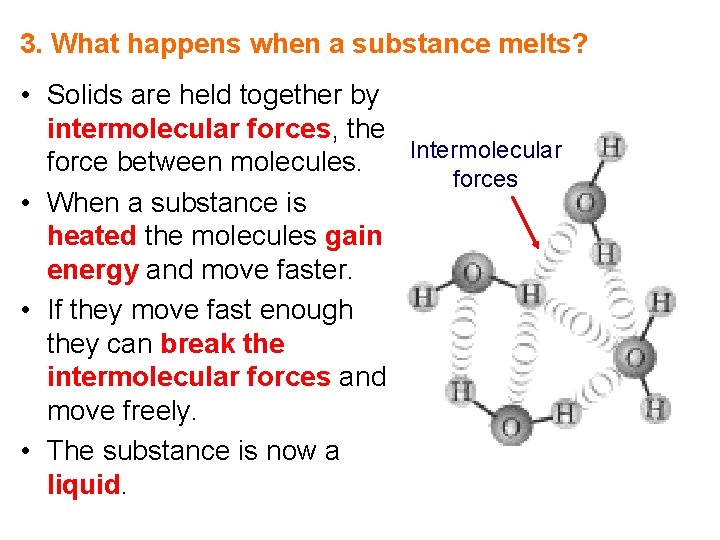
3. What happens when a substance melts? • Solids are held together by intermolecular forces, the Intermolecular force between molecules. forces • When a substance is heated the molecules gain energy and move faster. • If they move fast enough they can break the intermolecular forces and move freely. • The substance is now a liquid.
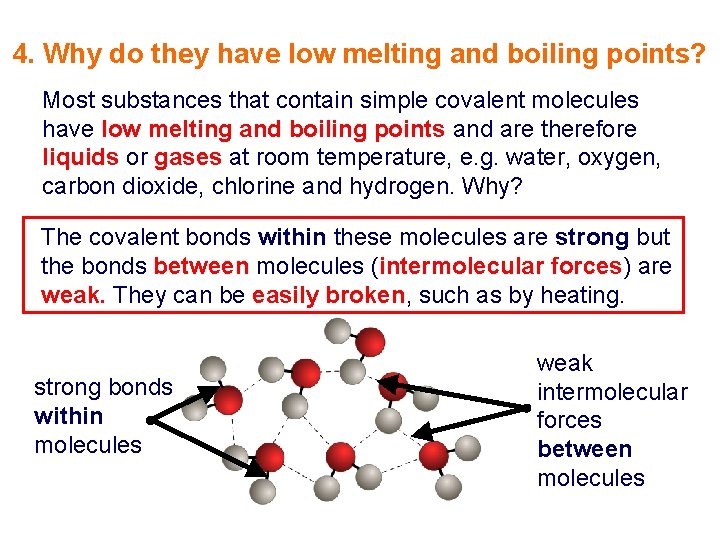
4. Why do they have low melting and boiling points? Most substances that contain simple covalent molecules have low melting and boiling points and are therefore liquids or gases at room temperature, e. g. water, oxygen, carbon dioxide, chlorine and hydrogen. Why? The covalent bonds within these molecules are strong but the bonds between molecules (intermolecular forces) are weak. They can be easily broken, such as by heating. strong bonds within molecules weak intermolecular forces between molecules
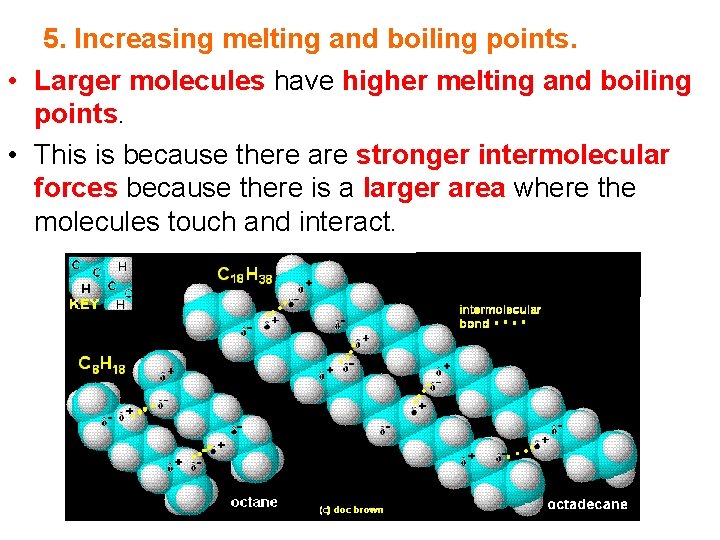
5. Increasing melting and boiling points. • Larger molecules have higher melting and boiling points. • This is because there are stronger intermolecular forces because there is a larger area where the molecules touch and interact.

6. Why do simple covalent molecules NOT conduct electricity? • Electricity is the movement of charged particles. • What are some examples of charged particles? o Electrons o Ions • Simple covalent molecules do NOT conduct electricity because they do not contain any charged particles.
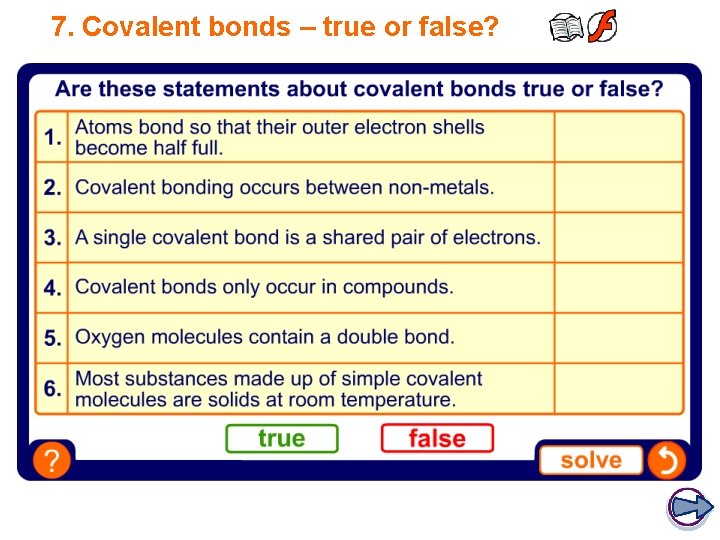
7. Covalent bonds – true or false? FALSE TRUE FALSE
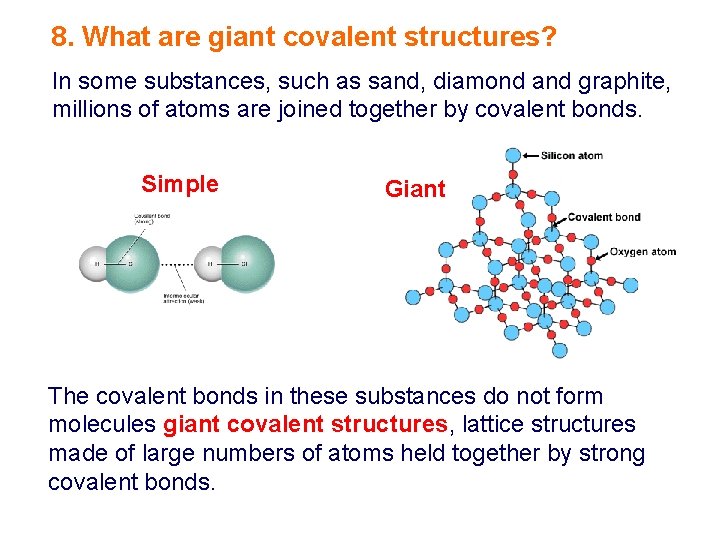
8. What are giant covalent structures? In some substances, such as sand, diamond and graphite, millions of atoms are joined together by covalent bonds. Simple Giant The covalent bonds in these substances do not form molecules giant covalent structures, lattice structures made of large numbers of atoms held together by strong covalent bonds.
9. Properties of giant covalent structures. • Giant covalent structures have very different properties from simple covalent structures because they contain strong covalent bonds (few intermolecular forces because it is all one giant lattice structure). • How do you think this would change the properties? solid a) Physical state? high b) Melting and boiling point? c) Strength? high d) Conducts electricity? no (one exception)
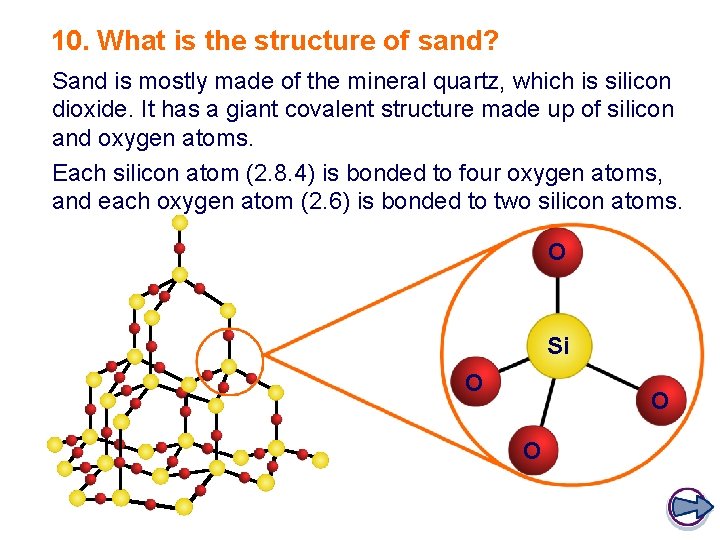
10. What is the structure of sand? Sand is mostly made of the mineral quartz, which is silicon dioxide. It has a giant covalent structure made up of silicon and oxygen atoms. Each silicon atom (2. 8. 4) is bonded to four oxygen atoms, and each oxygen atom (2. 6) is bonded to two silicon atoms. O Si O O O

11. Different forms of carbon Diamond and graphite appear to be very different substances but what do they have in common? Both diamond and graphite are made up of carbon atoms. These forms of carbon have different properties because the atoms are bonded in different arrangements which create different giant structures.
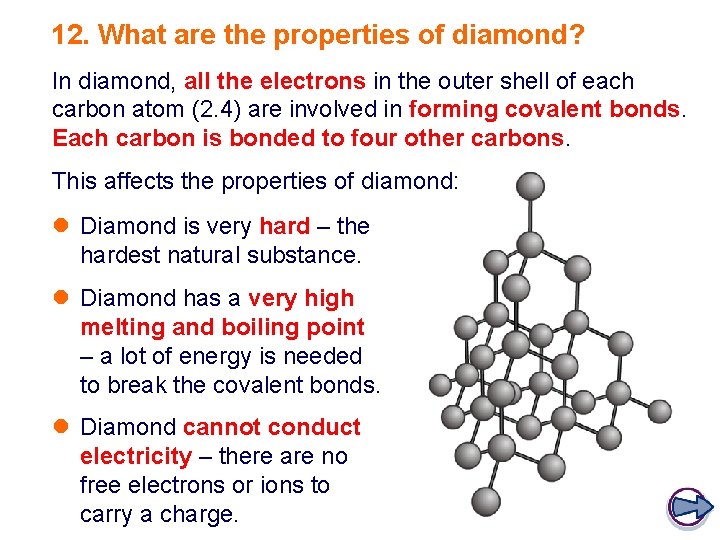
12. What are the properties of diamond? In diamond, all the electrons in the outer shell of each carbon atom (2. 4) are involved in forming covalent bonds. Each carbon is bonded to four other carbons. This affects the properties of diamond: l Diamond is very hard – the hardest natural substance. l Diamond has a very high melting and boiling point – a lot of energy is needed to break the covalent bonds. l Diamond cannot conduct electricity – there are no free electrons or ions to carry a charge.
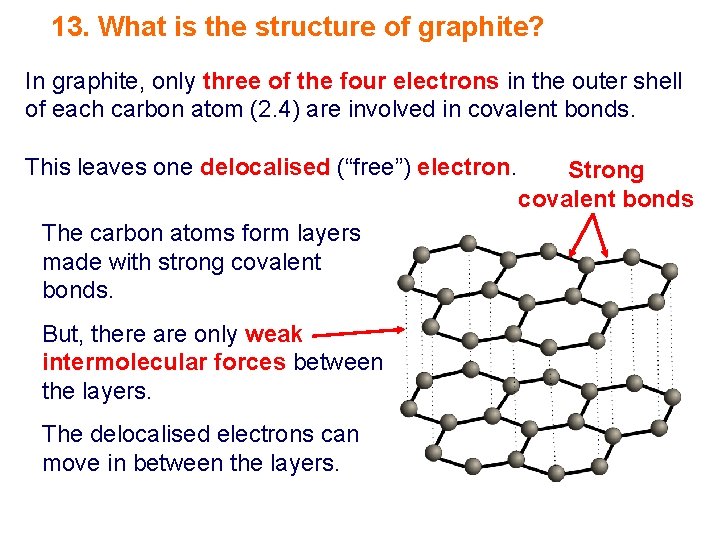
13. What is the structure of graphite? In graphite, only three of the four electrons in the outer shell of each carbon atom (2. 4) are involved in covalent bonds. This leaves one delocalised (“free”) electron. The carbon atoms form layers made with strong covalent bonds. But, there are only weak intermolecular forces between the layers. The delocalised electrons can move in between the layers. Strong covalent bonds
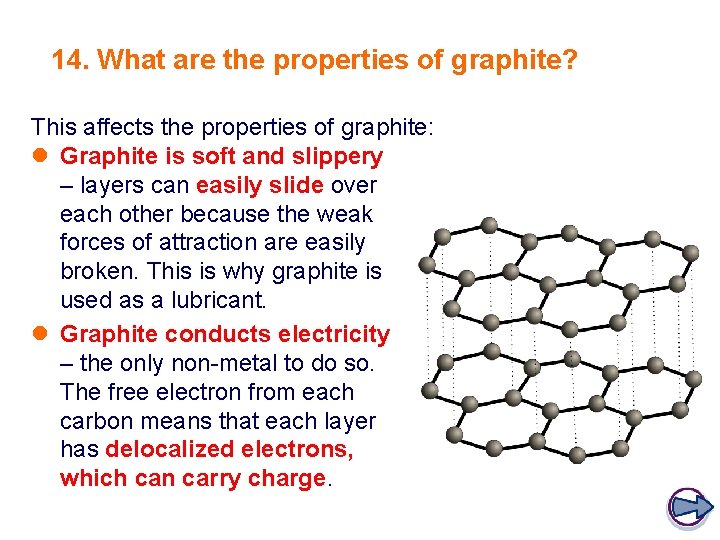
14. What are the properties of graphite? This affects the properties of graphite: l Graphite is soft and slippery – layers can easily slide over each other because the weak forces of attraction are easily broken. This is why graphite is used as a lubricant. l Graphite conducts electricity – the only non-metal to do so. The free electron from each carbon means that each layer has delocalized electrons, which can carry charge.
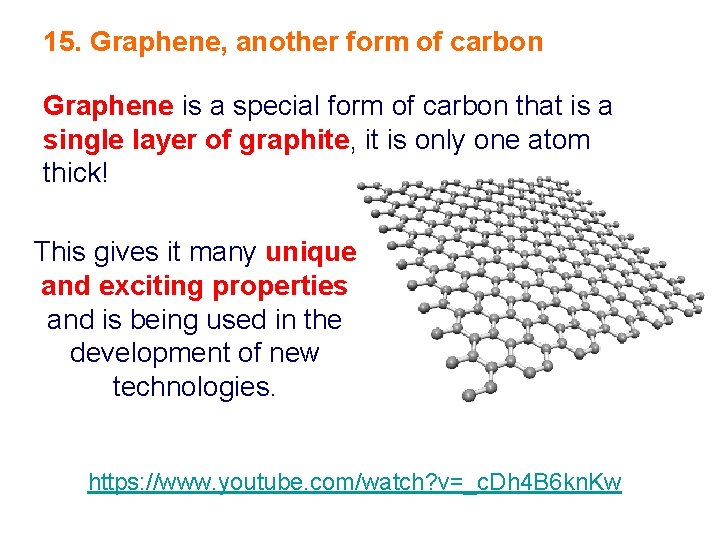
15. Graphene, another form of carbon Graphene is a special form of carbon that is a single layer of graphite, it is only one atom thick! This gives it many unique and exciting properties and is being used in the development of new technologies. https: //www. youtube. com/watch? v=_c. Dh 4 B 6 kn. Kw
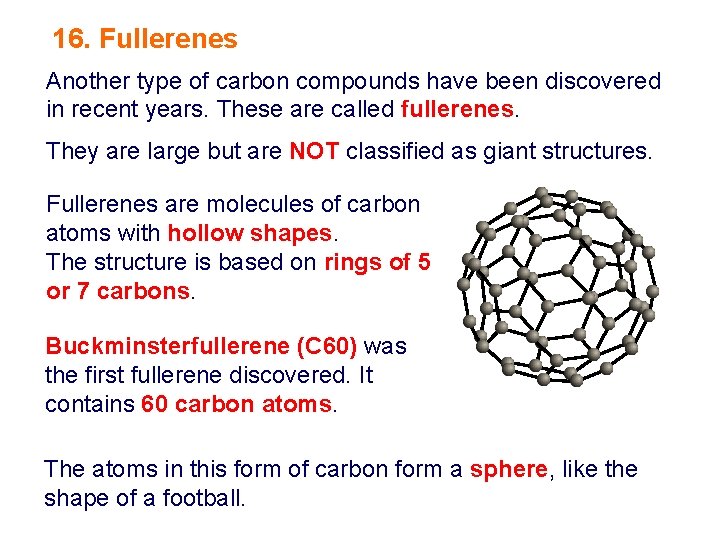
16. Fullerenes Another type of carbon compounds have been discovered in recent years. These are called fullerenes. They are large but are NOT classified as giant structures. Fullerenes are molecules of carbon atoms with hollow shapes. The structure is based on rings of 5 or 7 carbons. Buckminsterfullerene (C 60) was the first fullerene discovered. It contains 60 carbon atoms. The atoms in this form of carbon form a sphere, like the shape of a football.
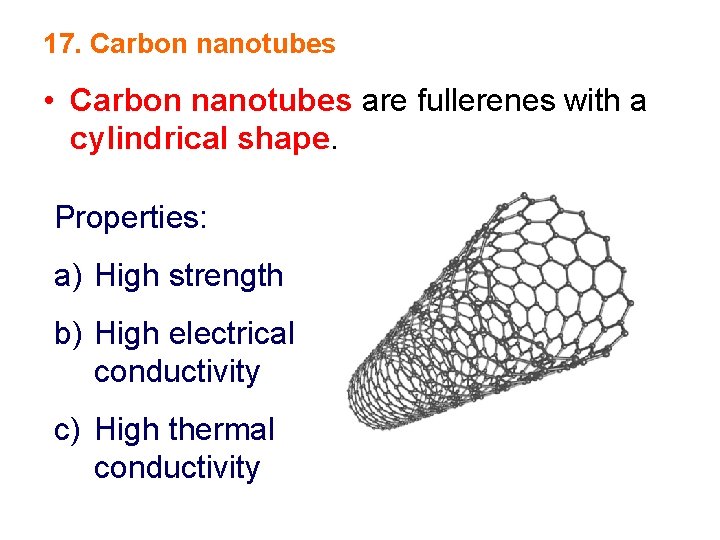
17. Carbon nanotubes • Carbon nanotubes are fullerenes with a cylindrical shape. Properties: a) High strength b) High electrical conductivity c) High thermal conductivity

Homework: Uses for fullerenes • Read the article about uses for fullerenes and write a short summary.
- Slides: 19