Properties of Carbon Atomic Structure Nucleus is made

Properties of Carbon

Atomic Structure • Nucleus is made up of positively charged protons and neutral neutrons • Negatively charged electrons orbit the nucleus

Atom Interactions • Atoms join together to make molecules • Two types of bonds: • Ionic-Atoms donate or accept electrons • Covalent-Atoms share electrons • *Covalent bonds are stronger than ionic bonds!
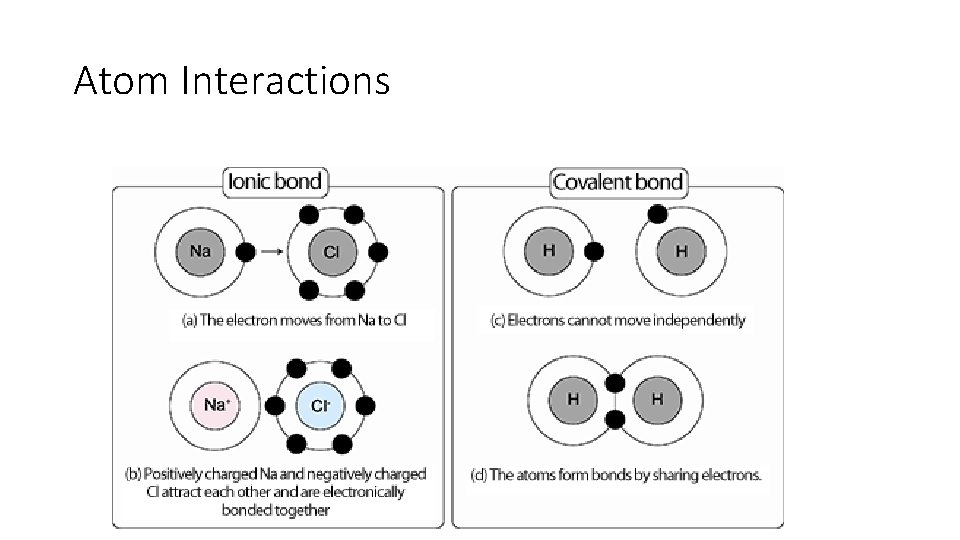
Atom Interactions
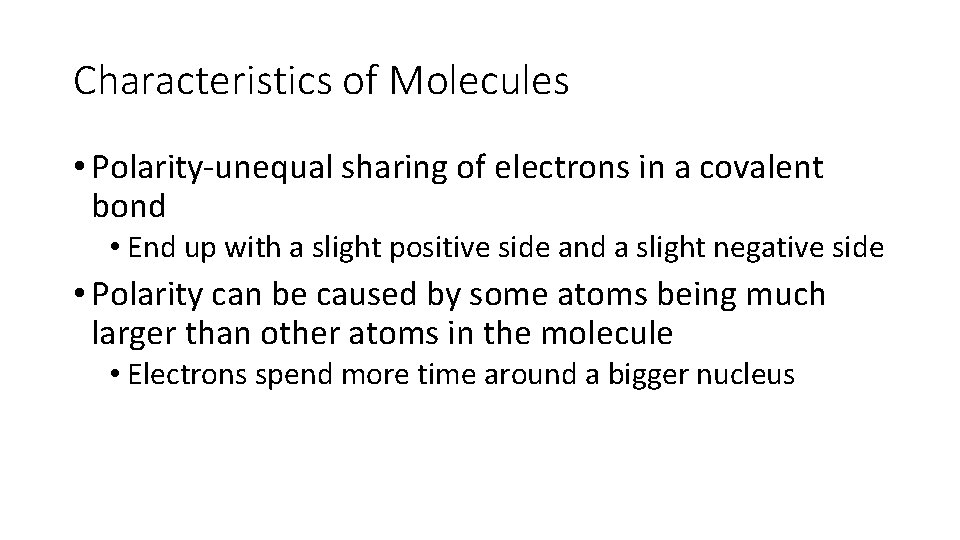
Characteristics of Molecules • Polarity-unequal sharing of electrons in a covalent bond • End up with a slight positive side and a slight negative side • Polarity can be caused by some atoms being much larger than other atoms in the molecule • Electrons spend more time around a bigger nucleus
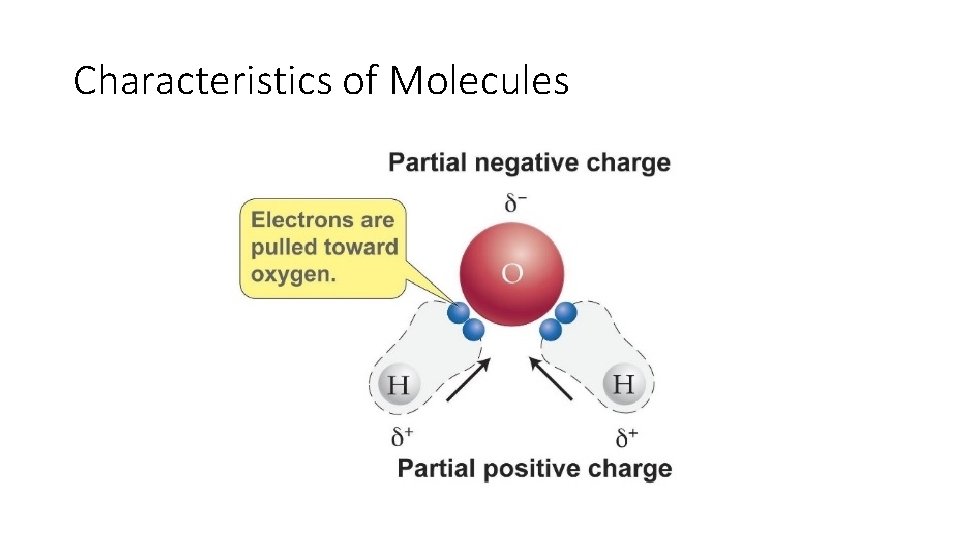
Characteristics of Molecules

Chemical Formula • There are 3 parts to a chemical formula: There are 3 Na and 6 O in this molecule

Chemical Formula • Molecule: 2 or more elements chemically bonded together. • Can be the same element or different elements • Symbols with 2 letters have an upper case followed by a lower case • Coefficient: big # in front of a compound or element • Subscript: Small number after an element or molecule
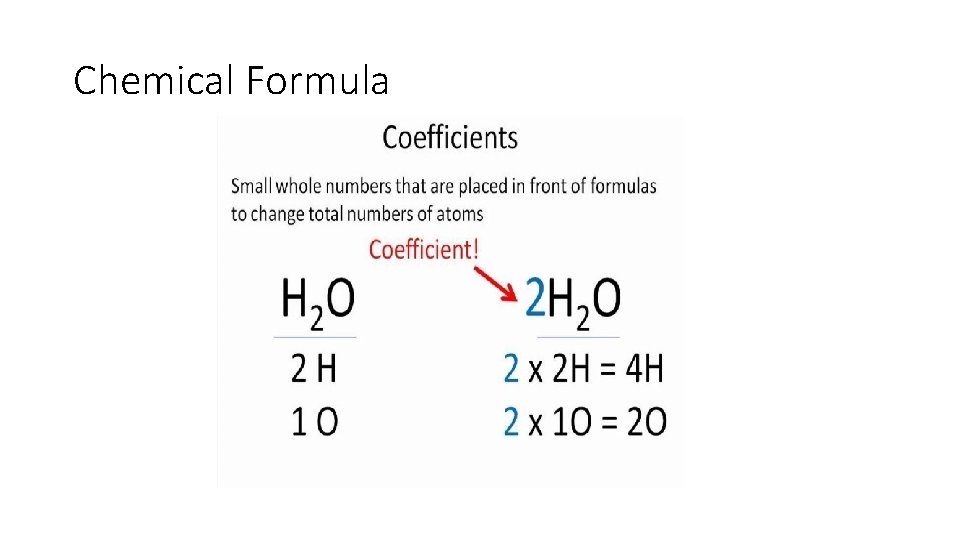
Chemical Formula
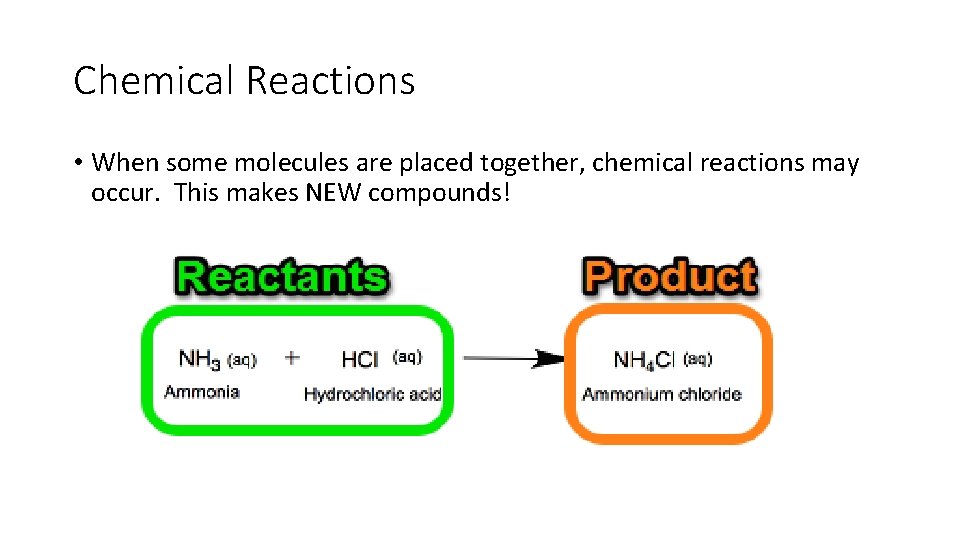
Chemical Reactions • When some molecules are placed together, chemical reactions may occur. This makes NEW compounds!

Chemical Reactions • Reactants-Molecules going INTO a reaction • Products-Molecules coming OUT of a reaction • Matter is neither created or destroyed! This means that your reactions must be balanced! • # of atoms on reactant side = # of atoms on product side

Balancing Chemical Equations

Carbon Properties • Most abundant atom in living things • Makes 4 strong covalent bonds • Allows for a variety of different shapes of molecules • Leads to a variety of functions • Makes up important biomolecules like carbohydrates, lipids, proteins, nucleic acids • Organic compounds

Carbon Properties • Most atoms want 4 valence electrons in their outer shell • Carbon has 4 which means it can make 4 more bonds to get to 8 • Can form rings, chains or branched molecules

Carbon in ALL Living Things • The 4 major biochemical compounds: • Carbohydrates • Proteins • Lipids • Nucleic Acids • They ALL contain carbon • They are involved in ALL life processes • Ex: using food for energy, giving structure to cells, transmitting information, storing genetic information

Biogeochemical Cycles • Recycle matter between organisms and the environment
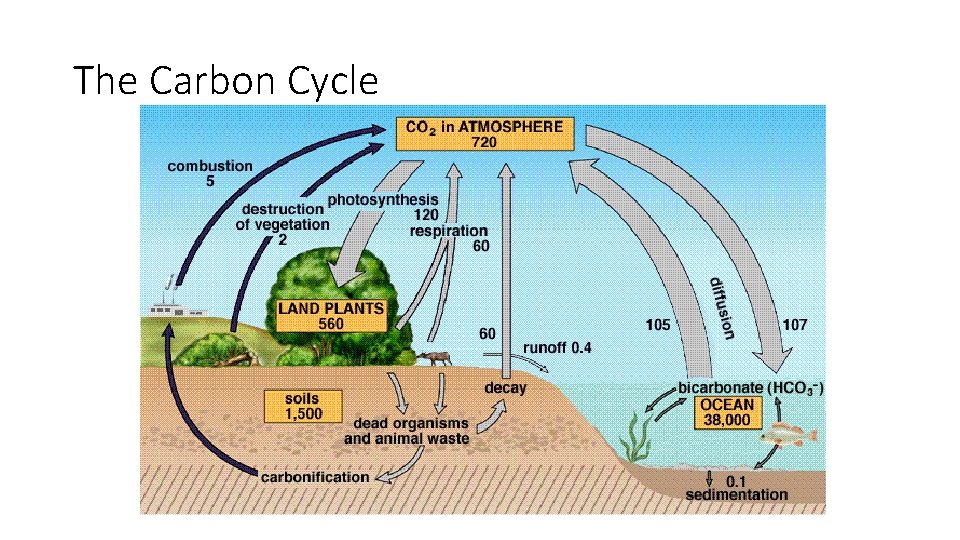
The Carbon Cycle
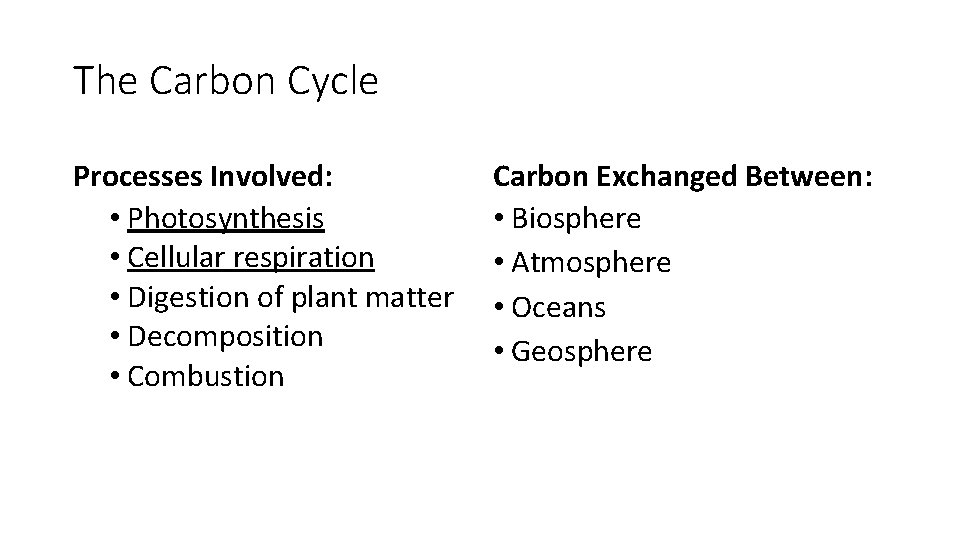
The Carbon Cycle Processes Involved: • Photosynthesis • Cellular respiration • Digestion of plant matter • Decomposition • Combustion Carbon Exchanged Between: • Biosphere • Atmosphere • Oceans • Geosphere

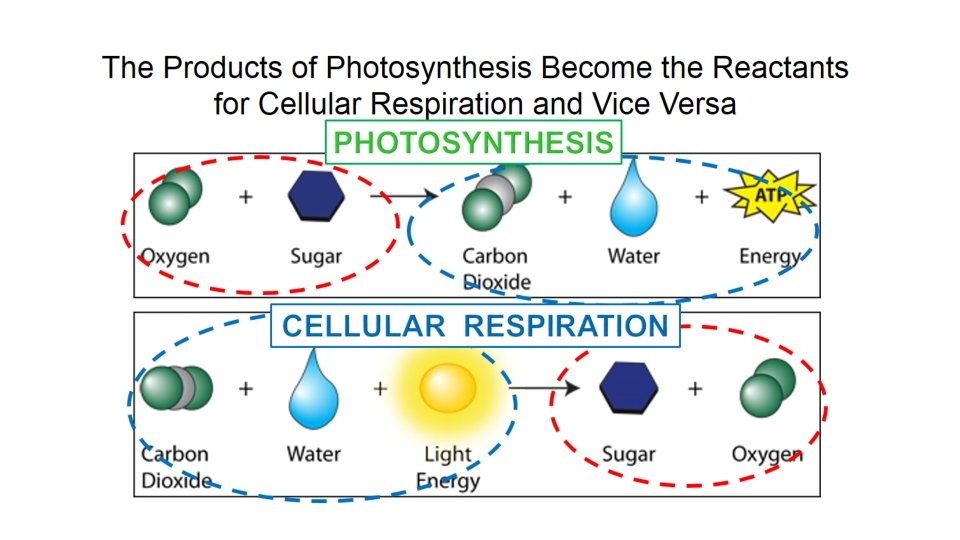
Photosynthesis & Cellular Respiration
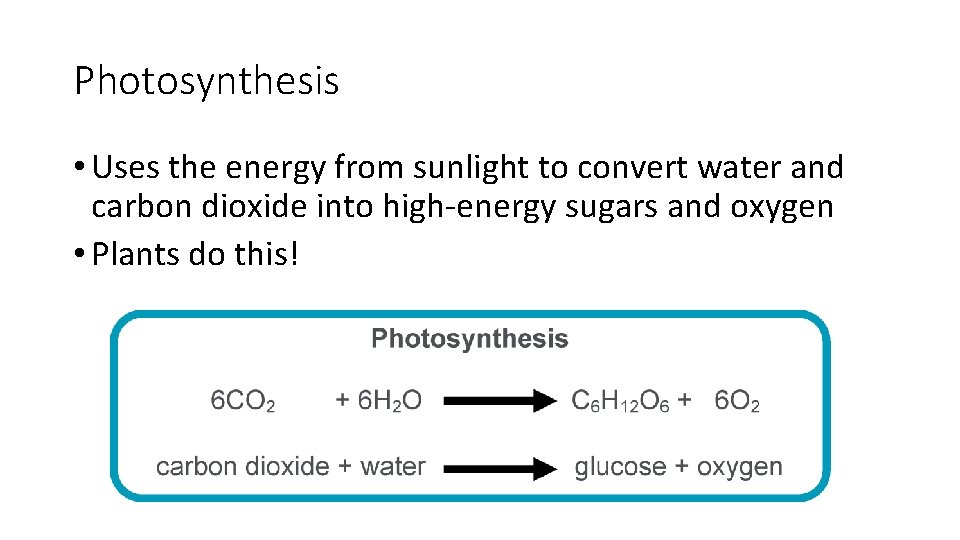
Photosynthesis • Uses the energy from sunlight to convert water and carbon dioxide into high-energy sugars and oxygen • Plants do this!

Cellular Respiration • Releases energy by breaking down glucose (or other food molecules) in the presence of oxygen • Responsible for most of our stored energy • All living things do this!
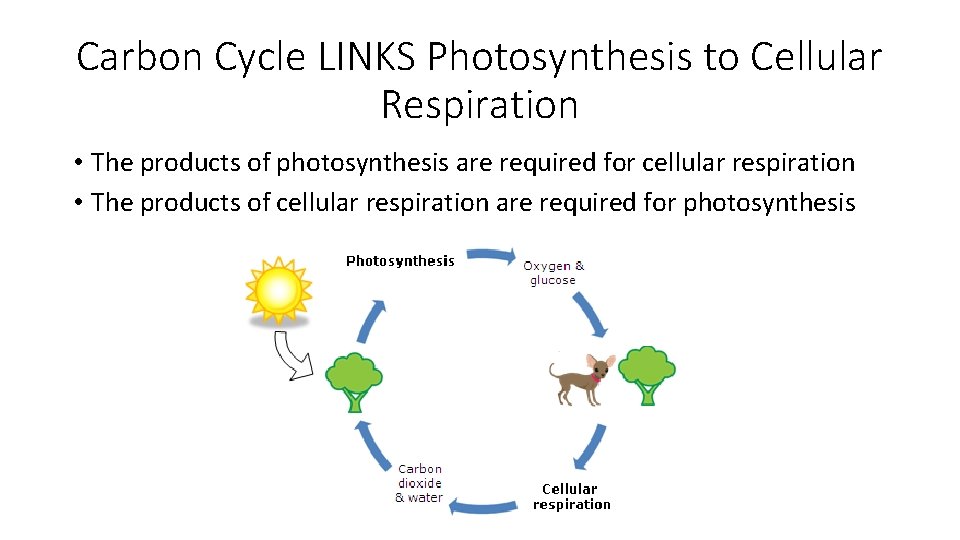
Carbon Cycle LINKS Photosynthesis to Cellular Respiration • The products of photosynthesis are required for cellular respiration • The products of cellular respiration are required for photosynthesis
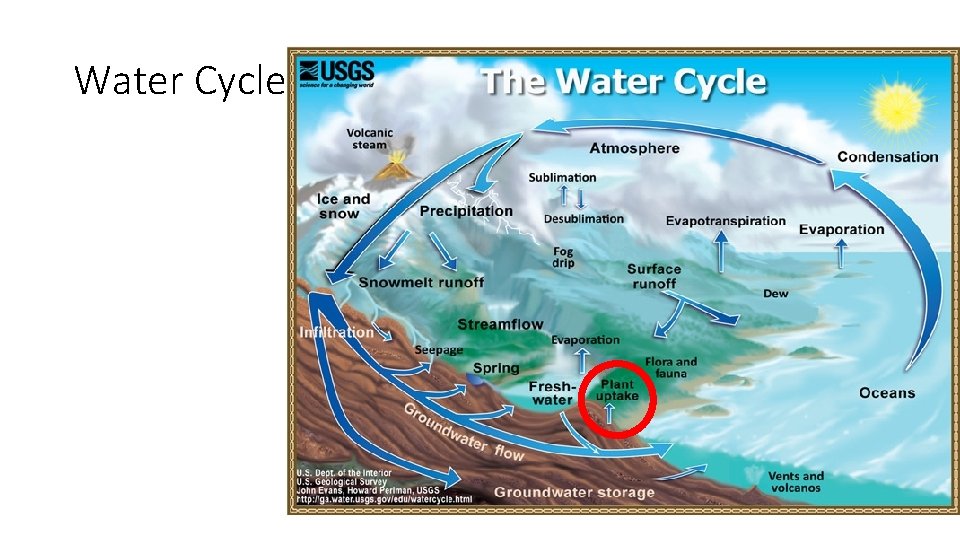
Water Cycle
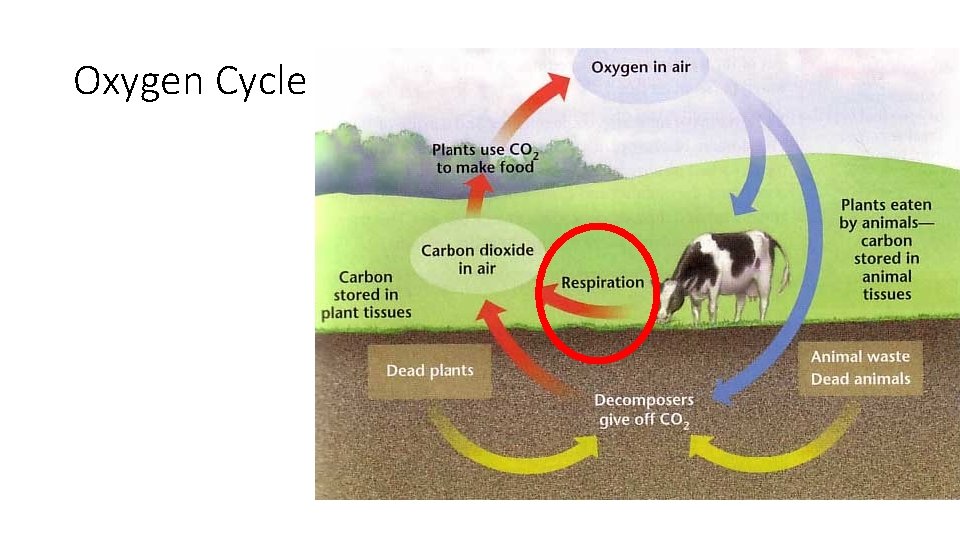
Oxygen Cycle
- Slides: 25