Properties of Bonds 1 Polarity 2 Bond Order

Properties of Bonds 1. Polarity 2. Bond Order 3. Bond Length 4. Bond Energy
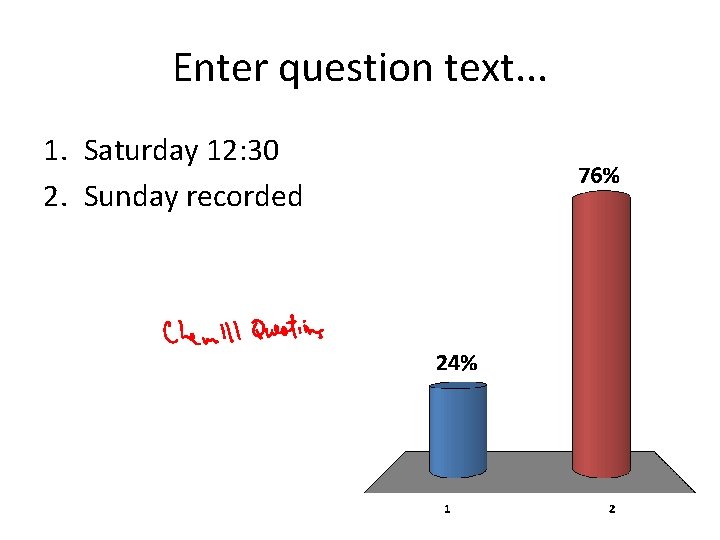
Enter question text. . . 1. Saturday 12: 30 2. Sunday recorded
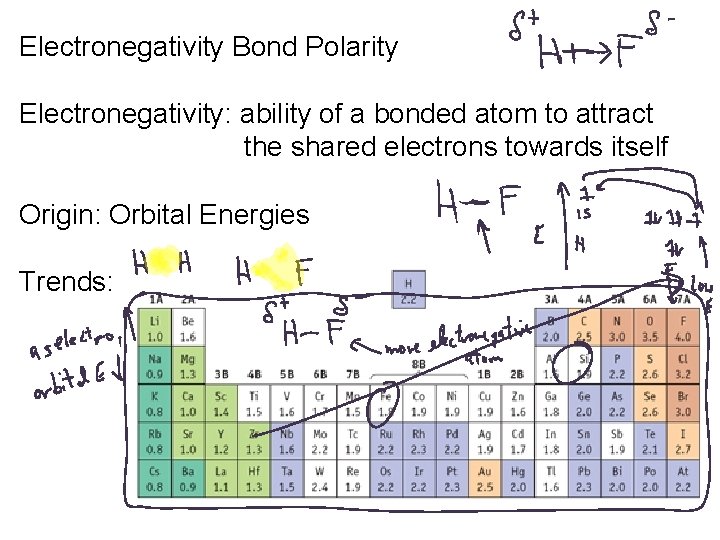
Electronegativity Bond Polarity Electronegativity: ability of a bonded atom to attract the shared electrons towards itself Origin: Orbital Energies Trends:
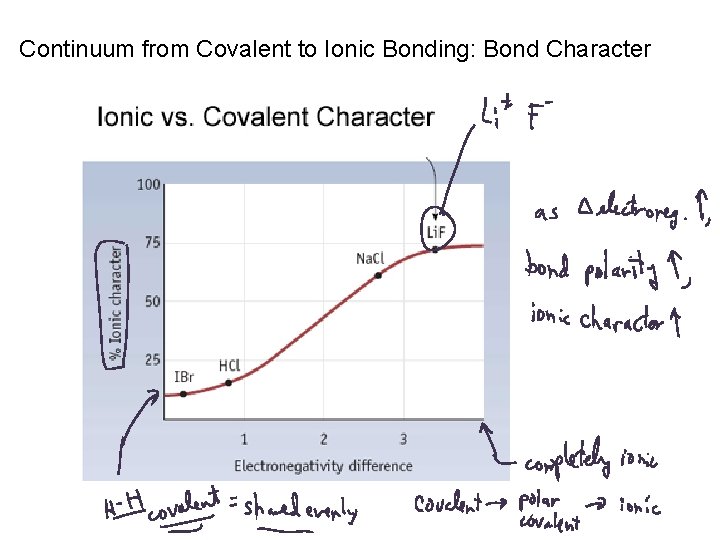
Continuum from Covalent to Ionic Bonding: Bond Character
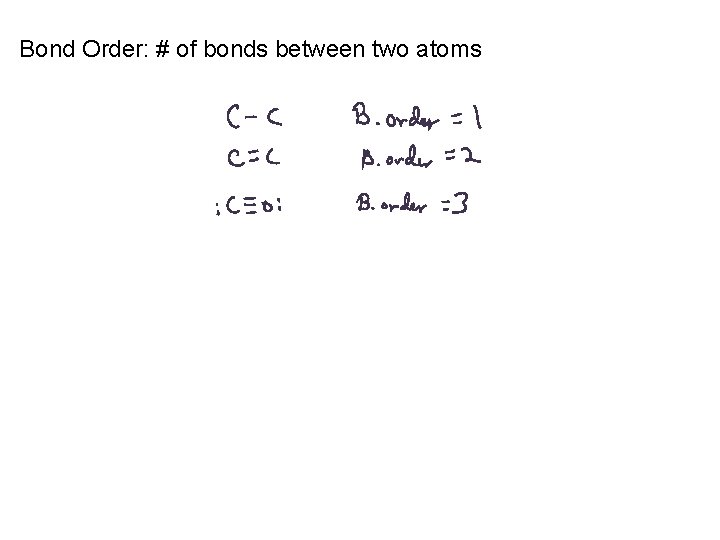
Bond Order: # of bonds between two atoms
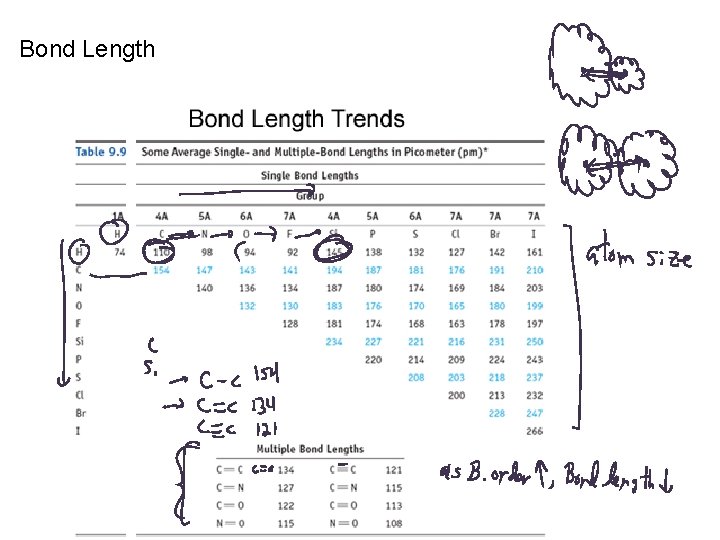
Bond Length
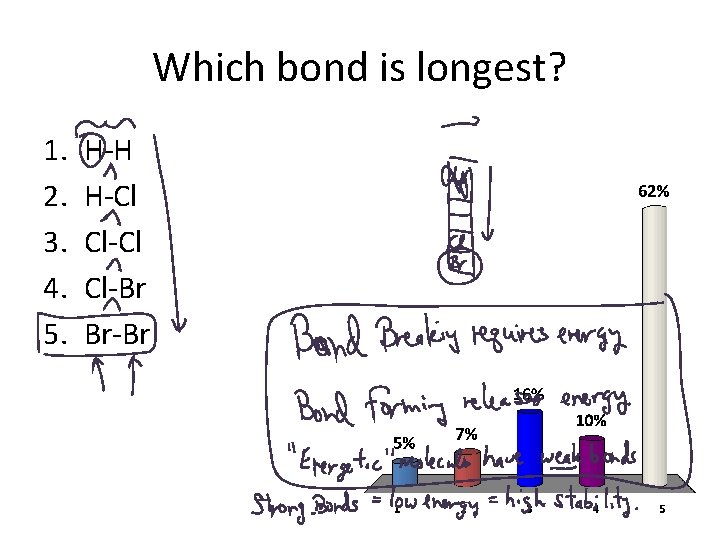
Which bond is longest? 1. 2. 3. 4. 5. H-H H-Cl Cl-Br Br-Br
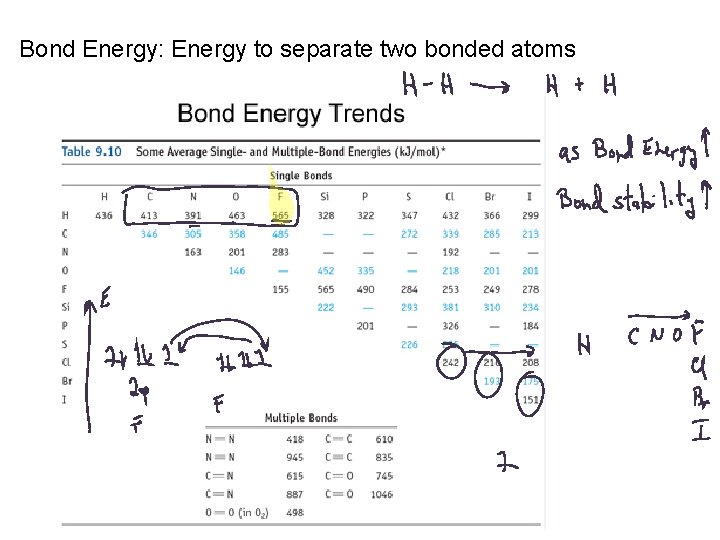
Bond Energy: Energy to separate two bonded atoms
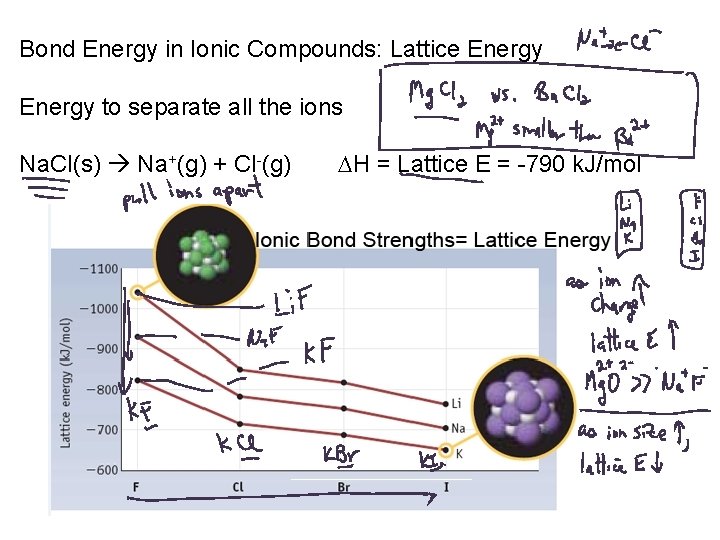
Bond Energy in Ionic Compounds: Lattice Energy to separate all the ions Na. Cl(s) Na+(g) + Cl-(g) H = Lattice E = -790 k. J/mol
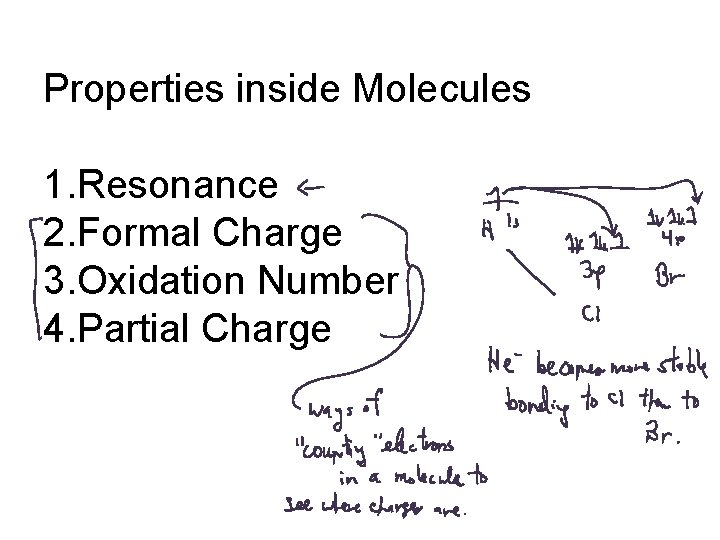
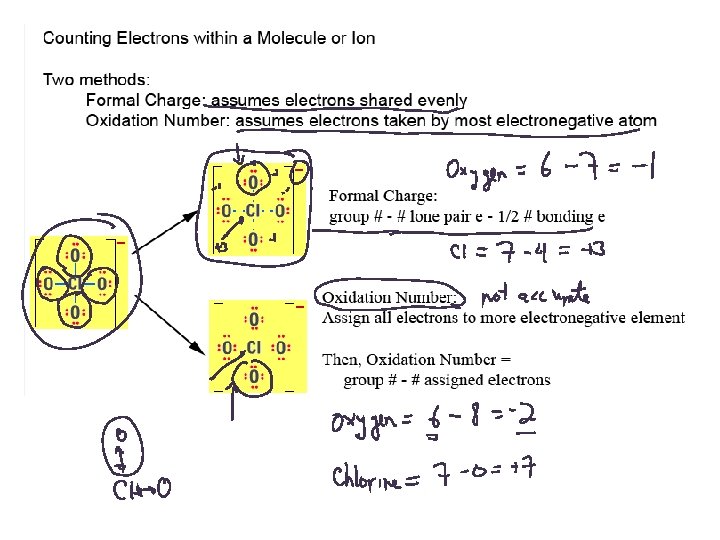
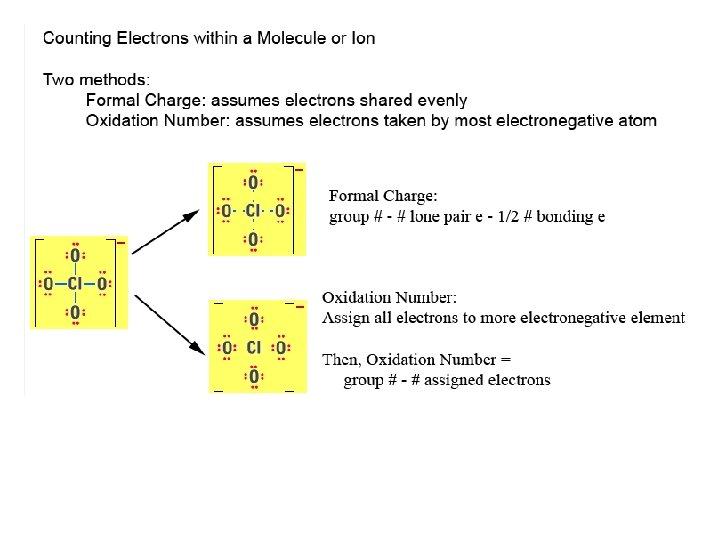
Properties inside Molecules 1. Resonance 2. Formal Charge 3. Oxidation Number 4. Partial Charge
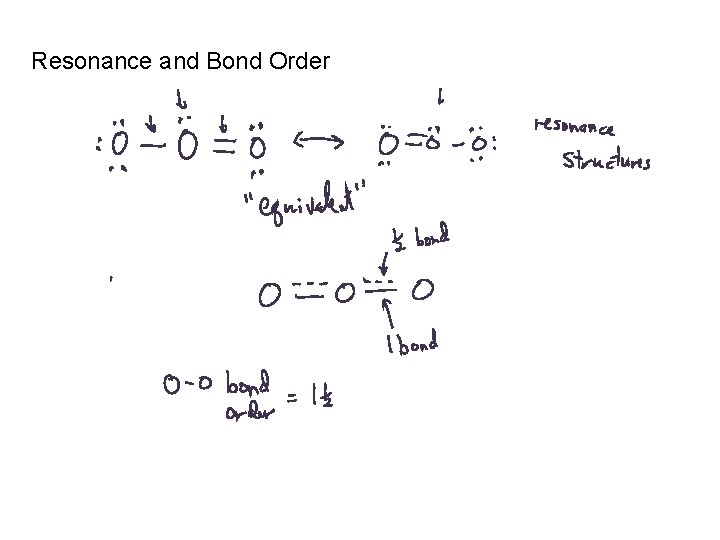
Resonance and Bond Order
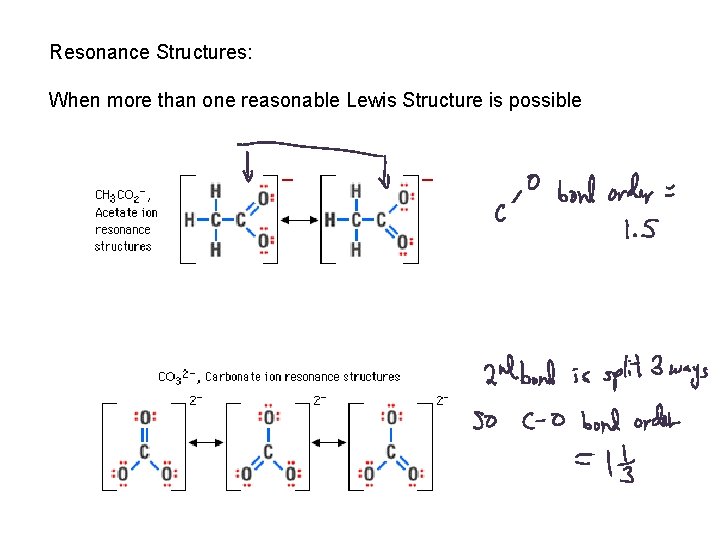
Resonance Structures: When more than one reasonable Lewis Structure is possible
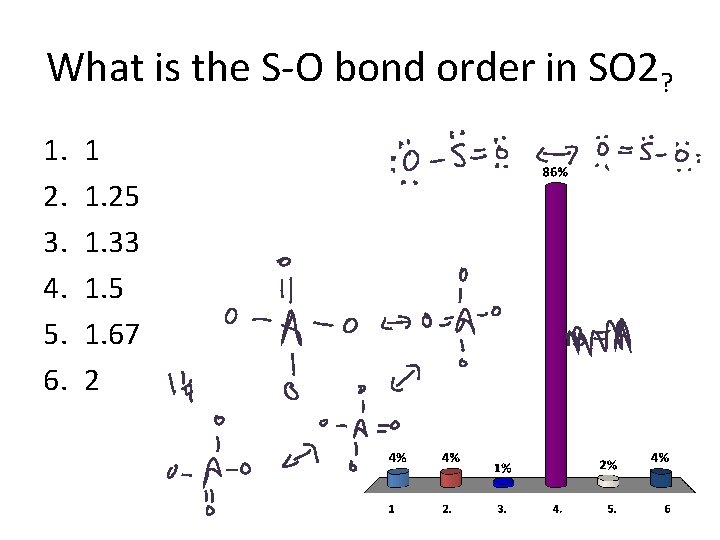
What is the S-O bond order in SO 2? 1. 2. 3. 4. 5. 6. 1 1. 25 1. 33 1. 5 1. 67 2
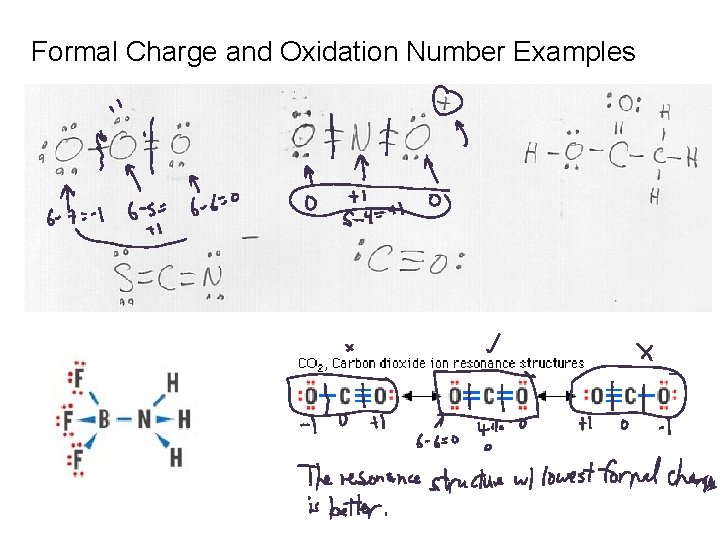
Formal Charge and Oxidation Number Examples
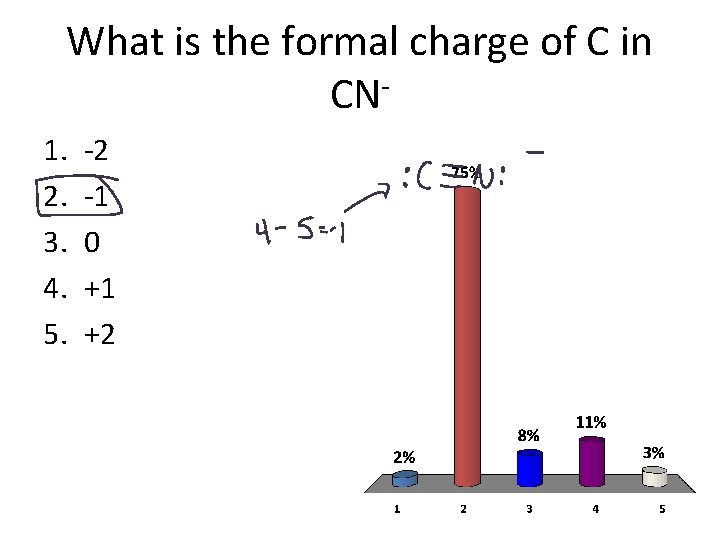
What is the formal charge of C in CN 1. 2. 3. 4. 5. -2 -1 0 +1 +2
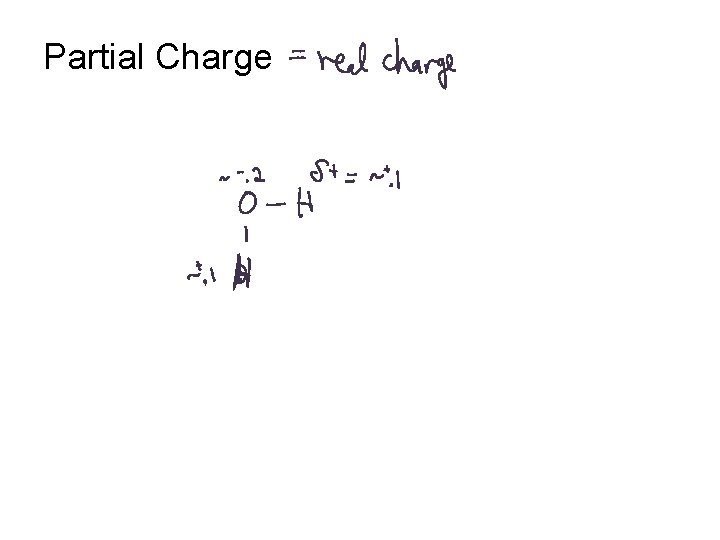
Partial Charge
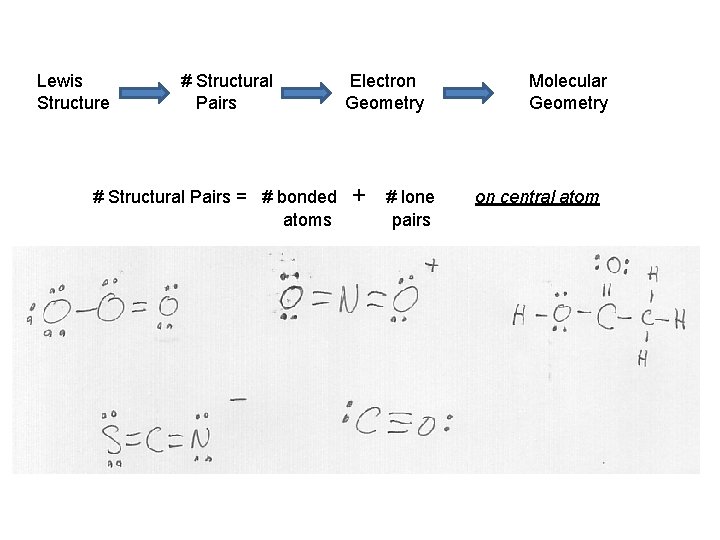
Lewis Structure # Structural Pairs = # bonded atoms Electron Geometry + # lone pairs Molecular Geometry on central atom
How many structural pairs in NH 3? 1. 2. 3. 4. 5. 6. 1 2 3 4 5 6
How many structural pairs in CO 2? 1. 2. 3. 4. 5. 6. 1 2 3 4 5 6
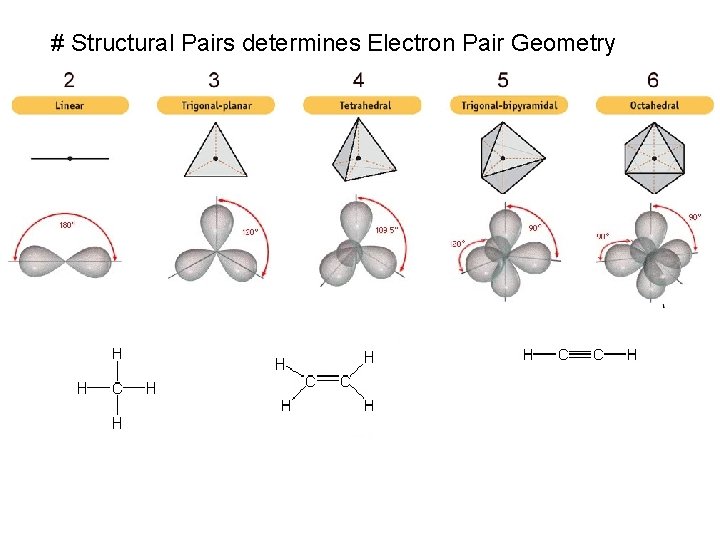
# Structural Pairs determines Electron Pair Geometry
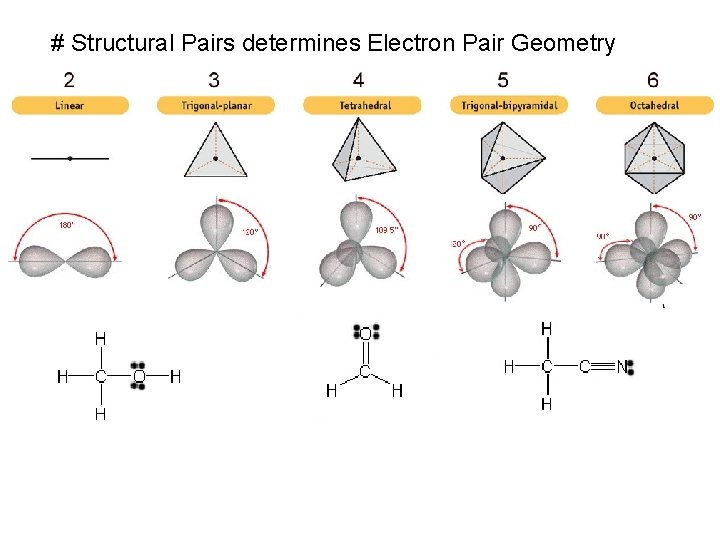
# Structural Pairs determines Electron Pair Geometry
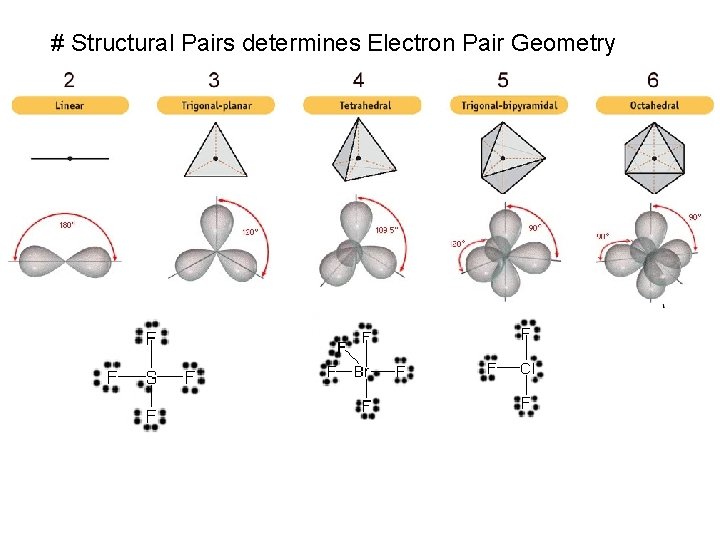
# Structural Pairs determines Electron Pair Geometry
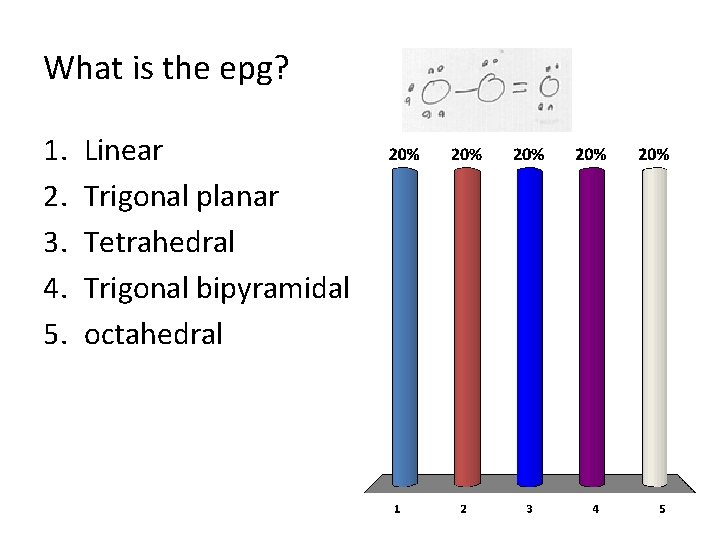
What is the epg? 1. 2. 3. 4. 5. Linear Trigonal planar Tetrahedral Trigonal bipyramidal octahedral
What is the epg? 1. 2. 3. 4. 5. Linear Trigonal planar Tetrahedral Trigonal bipyramidal octahedral
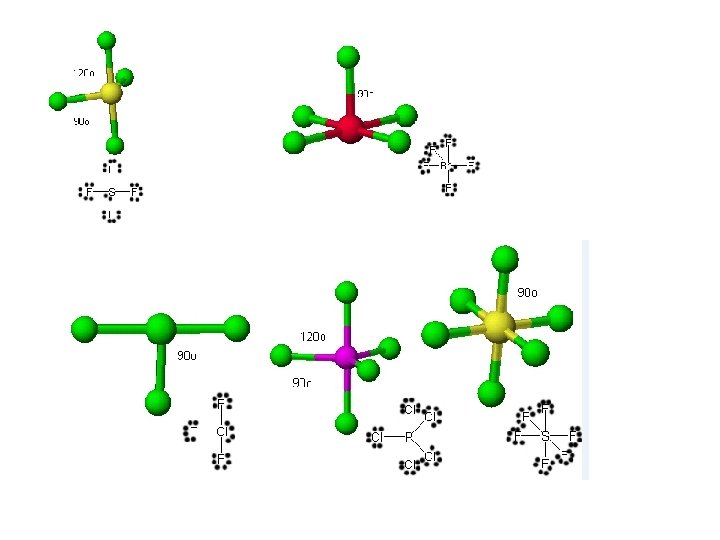
What is the epg? 1. 2. 3. 4. 5. SF 4 Linear Trigonal planar Tetrahedral Trigonal bipyramidal octahedral
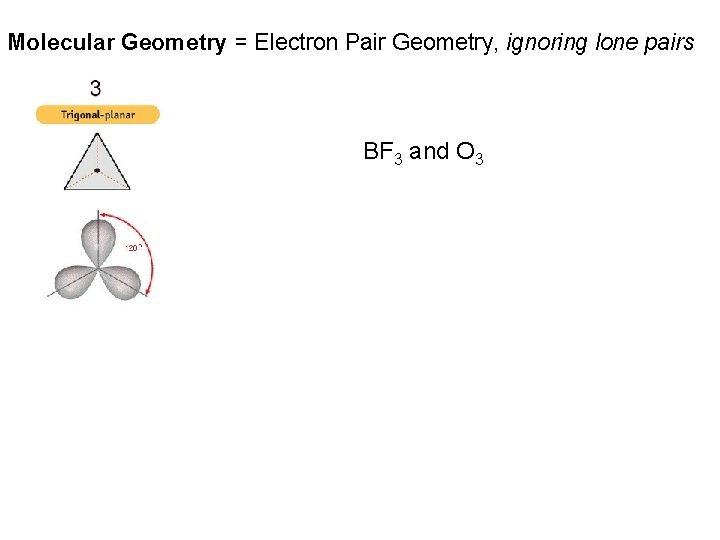
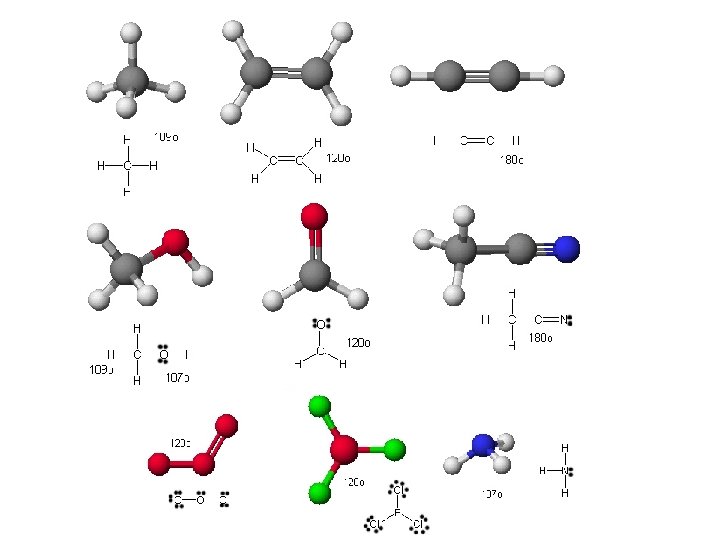
Molecular Geometry = Electron Pair Geometry, ignoring lone pairs BF 3 and O 3
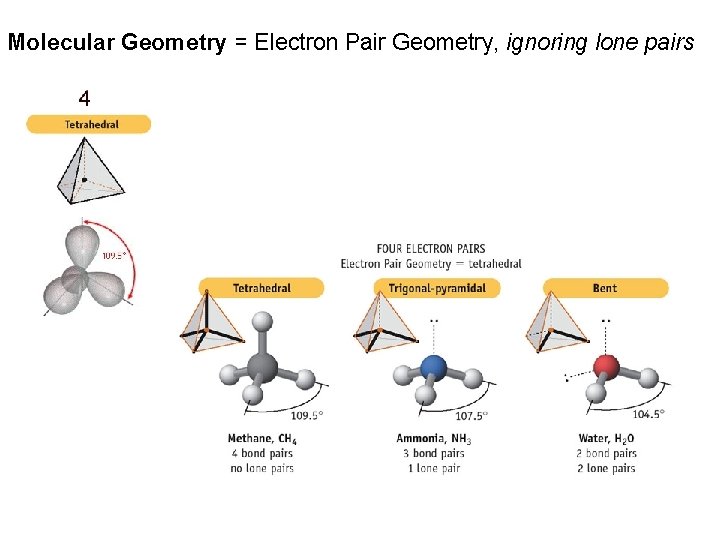
Molecular Geometry = Electron Pair Geometry, ignoring lone pairs
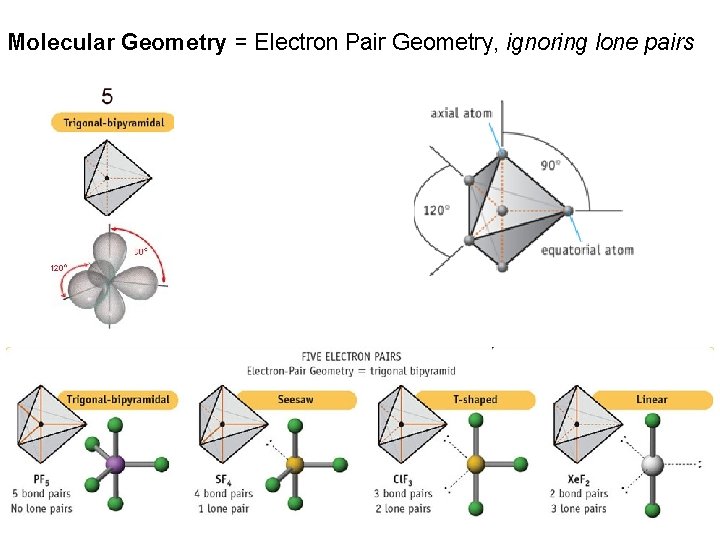
Molecular Geometry = Electron Pair Geometry, ignoring lone pairs

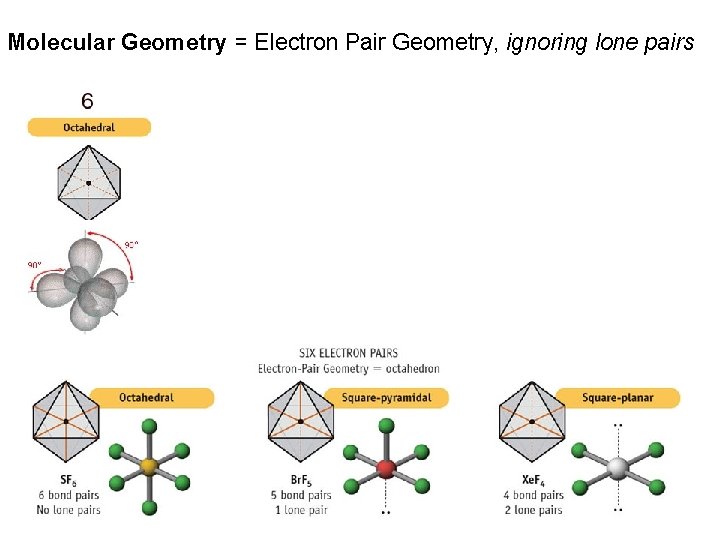
Molecular Geometry = Electron Pair Geometry, ignoring lone pairs
Molecular Geometry? 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral
Molecular Geometry? 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral
Molecular Geometry? 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral

Molecular Geometry? 1. 2. 3. 4. 5. 6. 7. 8. 9. Linear Bent Trigonal planar Tetrahedral Trigonal pyramid T-shaped Square pyramid Square planar octahedral

- Slides: 39