Properties of Acids and Bases Ionization Neutralization reactions
Properties of Acids and Bases Ionization Neutralization reactions Acid/base indicators
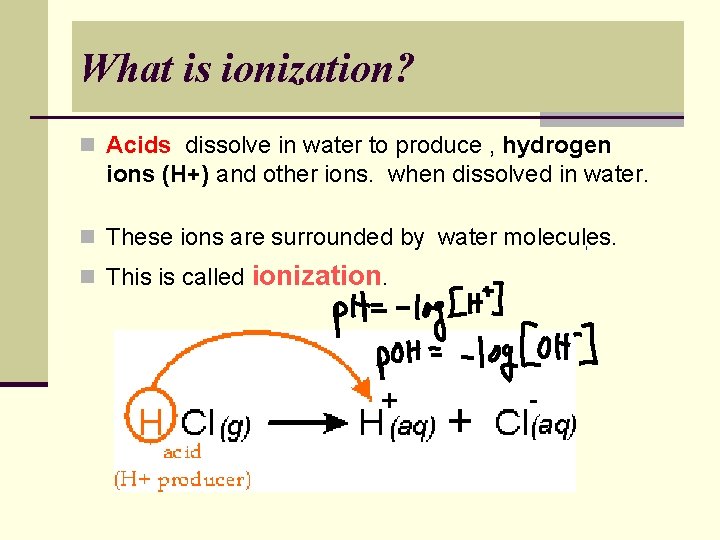
What is ionization? n Acids dissolve in water to produce , hydrogen ions (H+) and other ions. when dissolved in water. n These ions are surrounded by water molecules. n This is called ionization.
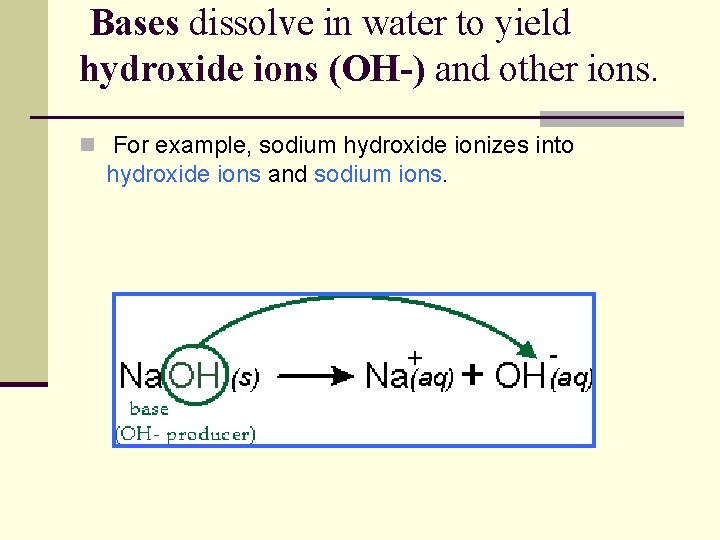
Bases dissolve in water to yield hydroxide ions (OH-) and other ions. n For example, sodium hydroxide ionizes into hydroxide ions and sodium ions.

Strong and Weak Acids & Bases n Strong acids ionize 100% in water: n n HCl, HNO 3, HCl. O 4, H 2 SO 4 Weak acids ionize less than 100% in water. Common examples: H 3 PO 4, HCH 3 COO, H 2 CO 3 Whether an acid is strong or weak depends on the amount of hydrogen ionized. Soluble metal hydroxides ionize 100% and are strong bases Na. OH, KOH, Mg(OH)2 Others are weak bases NH 3(aq) + H 2 O(l) NH 4+(aq) + OH-(aq)
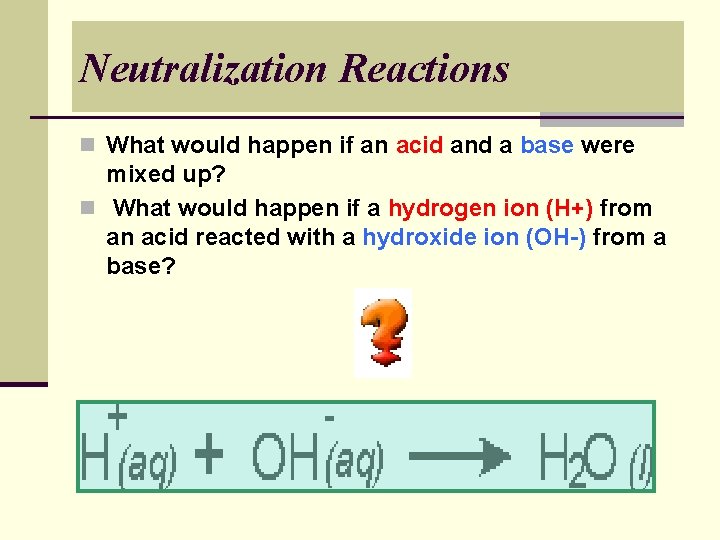
Neutralization Reactions n What would happen if an acid and a base were mixed up? n What would happen if a hydrogen ion (H+) from an acid reacted with a hydroxide ion (OH-) from a base?
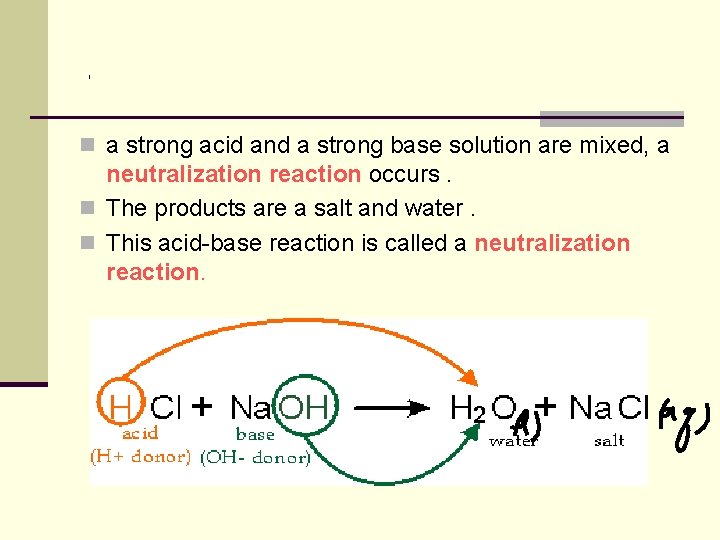
n a strong acid and a strong base solution are mixed, a neutralization reaction occurs. n The products are a salt and water. n This acid-base reaction is called a neutralization reaction.
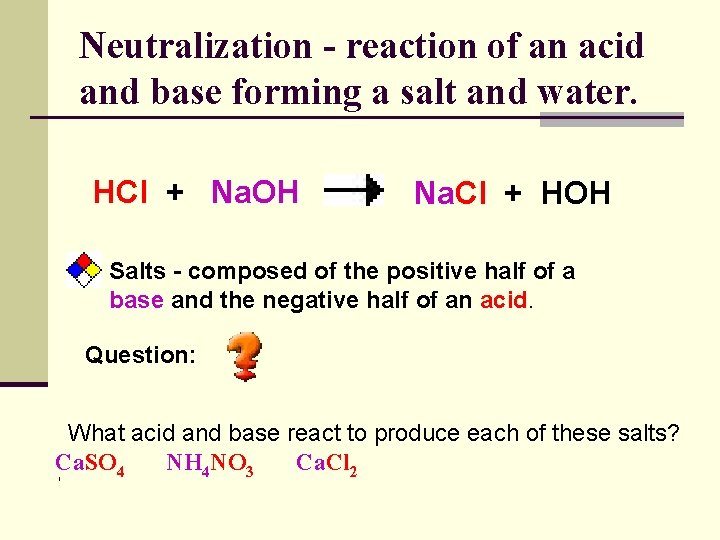
Neutralization - reaction of an acid and base forming a salt and water. HCl + Na. OH Na. Cl + HOH Salts - composed of the positive half of a base and the negative half of an acid. Question: What acid and base react to produce each of these salts? Ca. SO 4 NH 4 NO 3 Ca. Cl 2

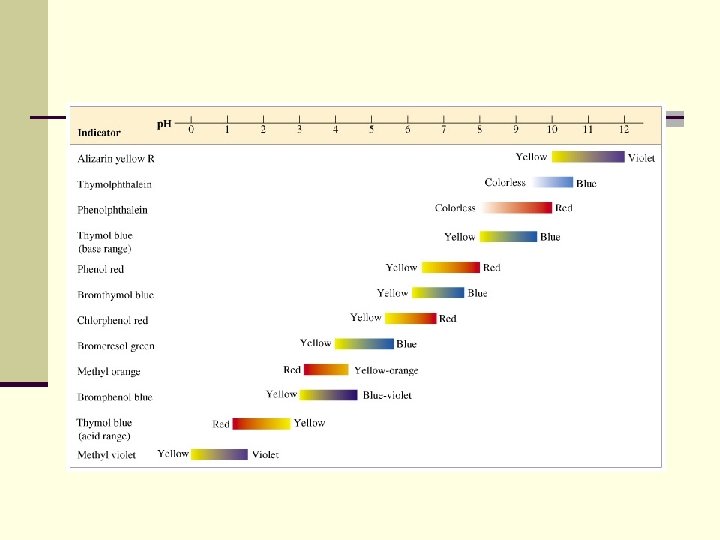
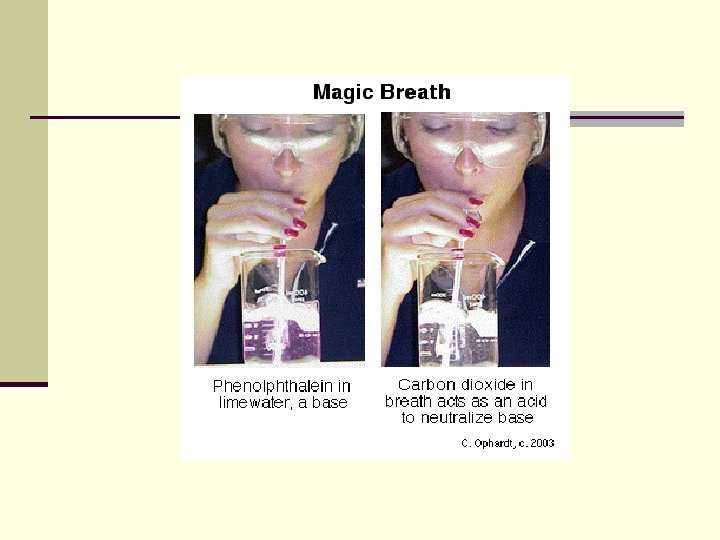
Acid-Base Indicators n Certain chemicals have the special property of appearing in different colours depending on the p. H of the solution they are in. n Such chemicals are known as acid-base indicators n A couple of drops of indicator in a solution will indicate its p. H.
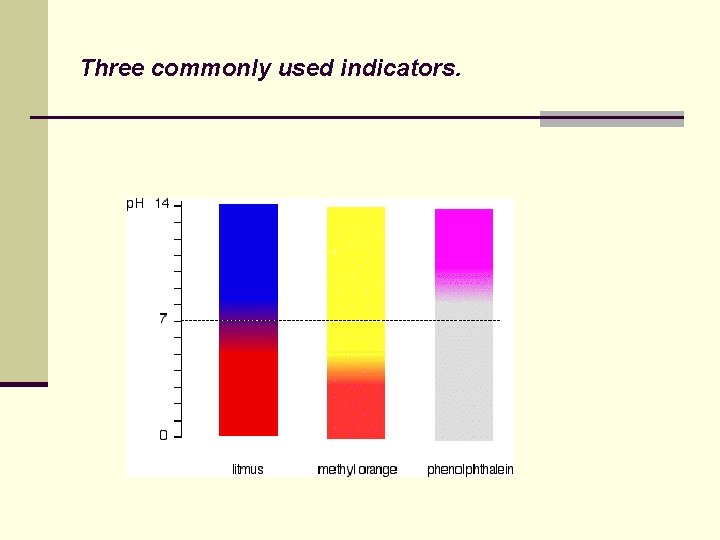
Three commonly used indicators.

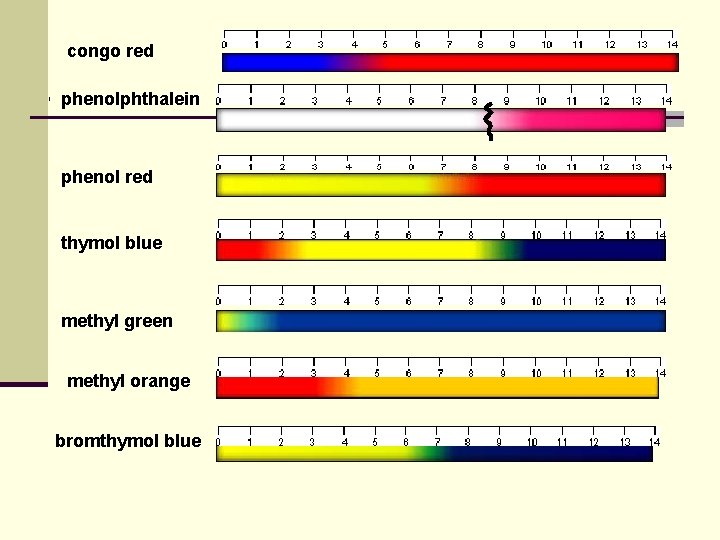
congo red phenolphthalein phenol red thymol blue methyl green methyl orange bromthymol blue

What household substances can be used as acid/base indicators • • • • • • · Beets change from red to purplish in very basic solution. · Blackberries, black currants, and black raspberries change from red in acids to dark blue or violet in basic solution. · Blue and red grapes contain several different p. H-sensitive anthocyanins. Red wines naturally contain these same pigments. · Blueberries change from blue (around p. H 2. 8 -3. 2) to red in a strongly acidic solution. · Carrots · Cherries and cherry juice is bright red in acidic solution but purple to blue in basic solution. · Cranberries · Curry powder and tumeric are spices that contain a bright yellow pigment called curcumin (which is not an anthocyanin). It turns from yellow at p. H 7. 4 to red at p. H 8. 6. · Geranium petals contain pelargonin, an anthocyanin which changes from orange-red in acid solution to bluish in basic solution. · Mood lipsticks undergo many interesting p. H-related color changes. · Morning glories contain an anthocyanin called "heavenly blue anthocyanin" which changes from purplish red at p. H 6. 6 to blue at p. H 7. 7. · Pansy petals · Petunia petals contain petunin, an anthocyanin that changes from reddish purple in acid to violet in basic solution. · Poppy flower petals · Red cabbage contains a mixture of anthocyanins and other pigments that indicate a wide range of p. H. · Red radish · Rhubarb · Rose petals contain the oxonium salt of cyanin, and they turn blue in basic solution. (The potassium or calcium salt of the same pigment makes cornflowers blue!) · Strawberries · Tea · Thyme (extract in alcohol) · Tulip petals · Vanilla extract, like onion, is an olfactory indicator. The vanilla odor isn't detectable in strongly basic solution because vanillin exists in ionic form at high p. H. · Violet petals
- Slides: 14