Properties and Classification of Matter Section A 1















- Slides: 19

Properties and Classification of Matter Section A 1. 2

CHEMISTRY!!!


http: //chemistry. about. com/od/everyday chemistry/ss/10 -Examples-of-Chemistry-in -Daily-Life. htm

Objectives distinguish between physical and chemical properties of substances classify matter as pure substances or mixtures and further into homogeneous or heterogeneous describe evidence for chemical changes

How do you classify a substance?

Classifying Substances Use two types of classification: 1) Physical properties Physical appearance and composition of a substance Ex. Boiling point 2) Chemical properties Reactivity of a substance Ex. Reaction with water

Physical or Chemical? For the next properties, classify as physical or chemical and then place into a chart within your notes Color? Solubility? Physical Ability to burn? Physical Chemical (combustion) Reaction to litmus? Chemical

Physical or Chemical? Flash point? Chemical (temp to ignite flame) State? physical Ductility? Physical Magnetism? Physical Reaction to heat? Chemical (melt or decompose? ) Crystal formation? Physical What did we miss on p 13, Figures A 1. 1 and A 1. 2? Fill in the rest of your chart with that information

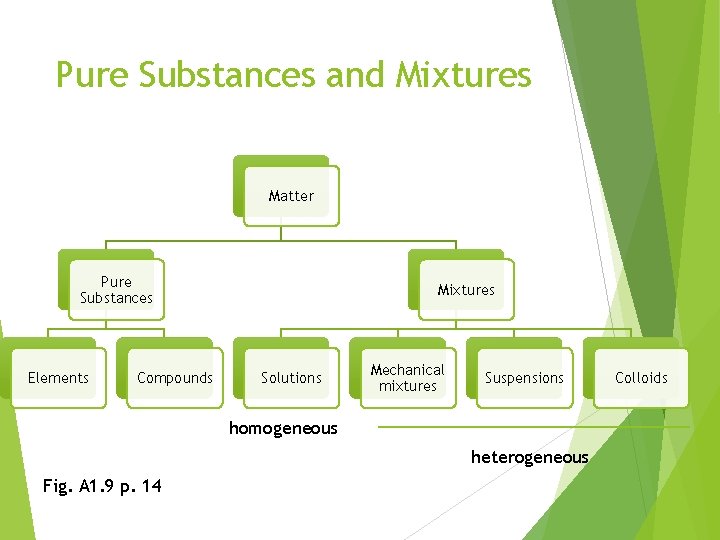
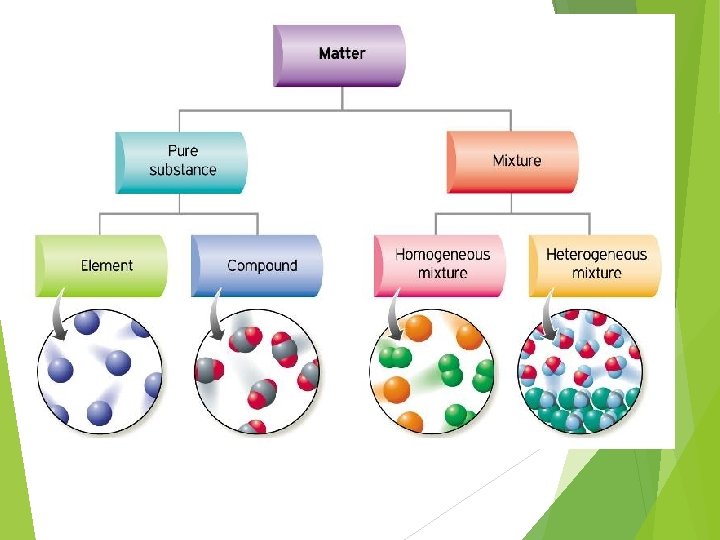
Pure Substances and Mixtures Matter Pure Substances Elements Compounds Mixtures Solutions Mechanical mixtures Suspensions homogeneous heterogeneous Fig. A 1. 9 p. 14 Colloids

Do Now Copy down Figure A 1. 9 on page 14 Define each term in the chart (except matter) and provide an example for each


Pure Substances and Mixtures Pure substances All particles making up substance are identical Physical and chemical properties constant Ex. element/compound Mixtures Combination of pure substances Properties vary with composition Elements Pure substance that cannot be broken down One type of atom (ex. Helium) Compounds Chemical combination of 2 or more elements in specific ratio Ex. water

Continued… Solution Separate components not visible (homogeneous) One dissolved into another Ex. Juice Mechanical mixtures Different substances visible (heterogeneous) Ex. Soil Suspension Components in different states Ex. Mud (dirt in water) Colloids Can’t easily separate the suspended substance Ex. milk

What is a chemical reaction?

Chemical Reactions Chemical change/chemical reaction Process that occurs when something reacts to form something new New substance has own physical and chemical properties Energy always absorbed or released How do we know that a chemical reactions has occurred? What signs do we look for?

Demo- Combustible Bubbles How do we know a chemical reaction has occurred? What do we see? Has there been a transfer of energy? How do we know that?

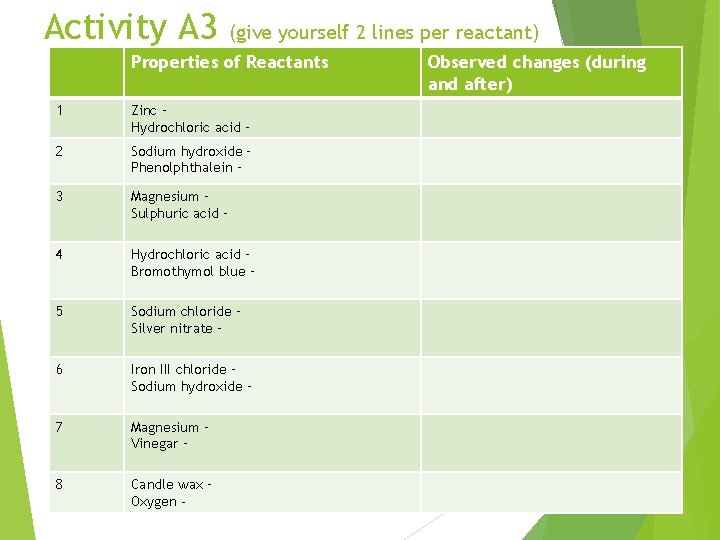
ACTIVITY A 3 – Evidence of chemical change Problem: What observable changes happen when a chemical reaction occurs? Materials, Procedure: see p 16 Observations: Prepare a data table that includes space for the following reactions. For each reactant, record its observable characteristics before the reaction. Also record changes that you observe when you combine the reactants. Give your table a title.

Activity A 3 (give yourself 2 lines per reactant) Properties of Reactants 1 Zinc – Hydrochloric acid – 2 Sodium hydroxide – Phenolphthalein – 3 Magnesium – Sulphuric acid – 4 Hydrochloric acid – Bromothymol blue – 5 Sodium chloride – Silver nitrate – 6 Iron III chloride – Sodium hydroxide – 7 Magnesium – Vinegar – 8 Candle wax – Oxygen - Observed changes (during and after)