Properties and Change Types of Substances Element made

Properties and Change

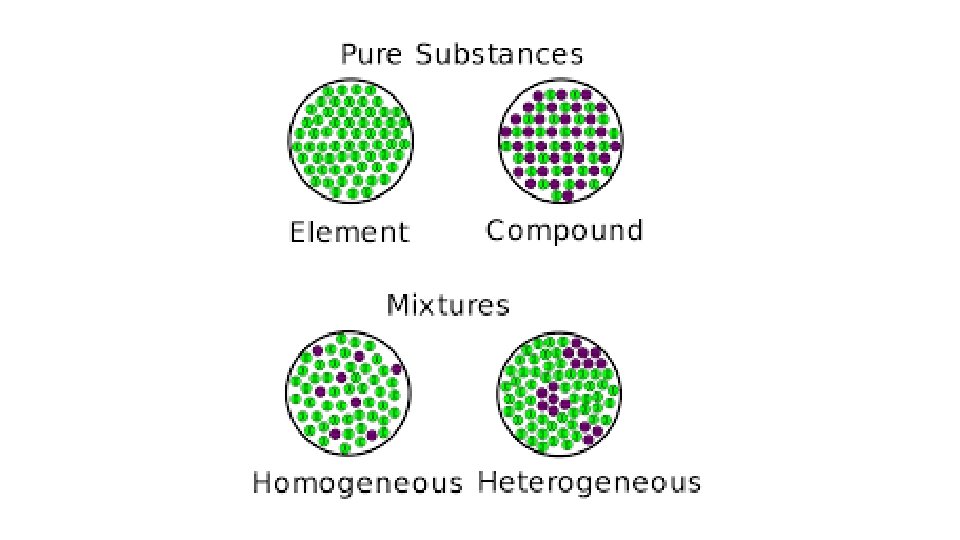
Types of Substances • Element – made of only one type of atom • Compound – made of two or more atoms that chemically combined, can be same elements bonded together or multiple elements

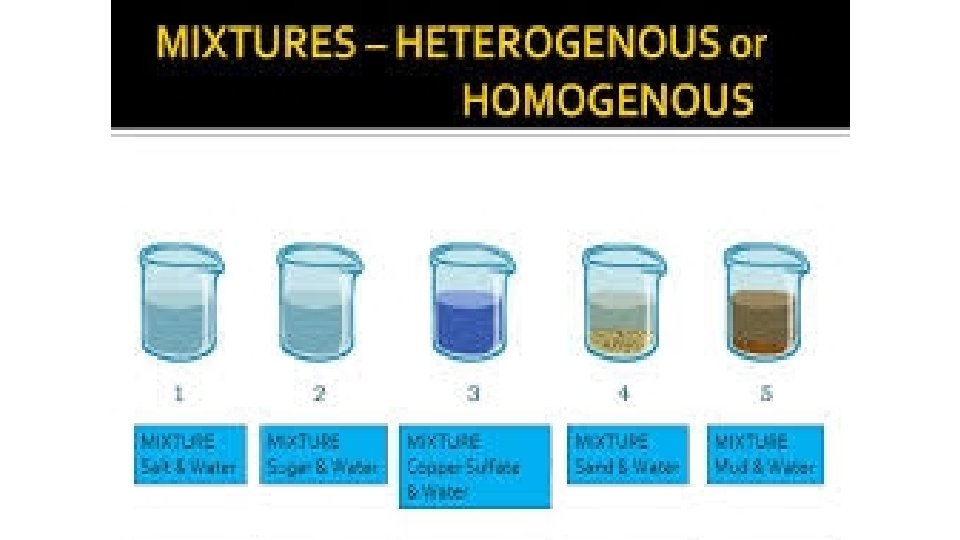
Types of Mixtures: • Homogeneous mixture – 2 or more substance physically combined where the phases (parts) of the mixture cannot be seen – the mixture appears the same through out (Gatorade) -Use distillation to separate • Heterogeneous mixture – 2 or more substance physically combined where the phases of the mixtures can be seen – it’s easily separated (oil and vinegar) - Use filtration to separate
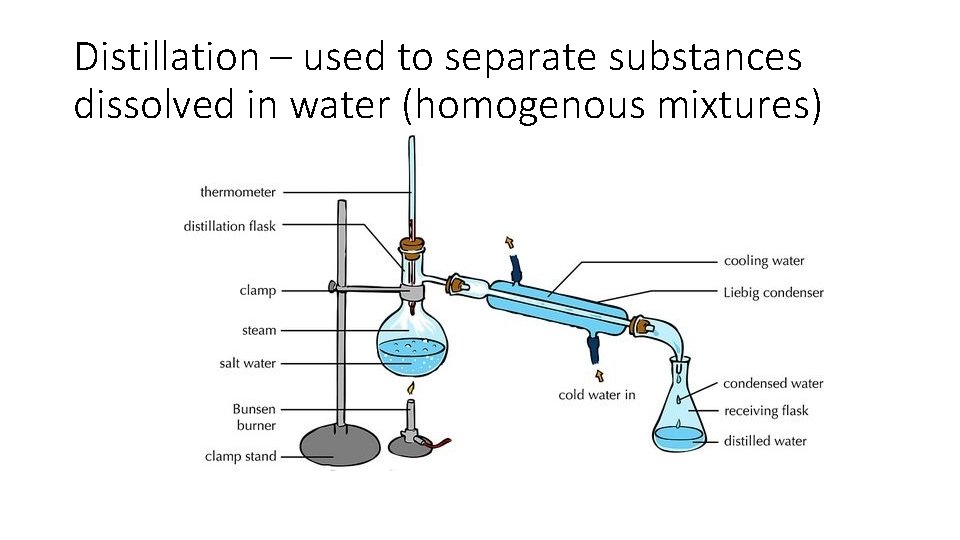
Distillation – used to separate substances dissolved in water (homogenous mixtures)
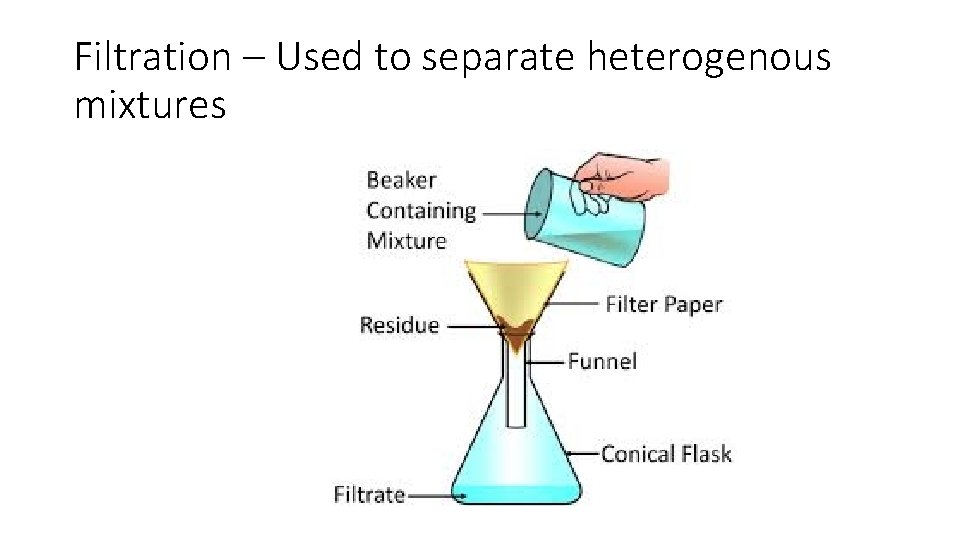
Filtration – Used to separate heterogenous mixtures

Properties: • Physical properties – property that can be observed at ANY time and do not depend on amount present. Usually a description of the substance • Examples – color, odor, texture, state of matter, density, malleability, buoyancy, magnetism, conductivity, solubility in water, boiling point, melting point • Chemical properties – can only be observed during a chemical reaction • Examples – does the substance react with; water, acid, or air • Is the substance flammable

Changes: • Physical change – any change that does not change the chemical make-up of the substance. Sometimes referred to as reversible. • Examples; cutting, coloring • Chemical Change – any change that changes the chemical make-up – a new compound has been formed or a compound has been broken into its elements.

Signs of a chemical change • color change – the substance has changed color as a result of the reaction and it has NOT been colored • Formation of a new gas – a gas has been produced by the reaction and not a vapor. One has not heated a substance in order for it to change into a gas, but a gas has formed (example the gas that resulted from the calcium in water) • Evolution of heat or light - heat has been produced by the reaction or a flame has been produced • Formation of a solid (Precipitate) – when two substances combine and a solid forms – not because the substance has frozen the solid or precipitate is a result of the reaction
- Slides: 10