PROJECT AND CAPABILITIES OVERVIEW About us Vision To

PROJECT AND CAPABILITIES OVERVIEW
About us Vision To be an integrated CRO with global distinction, aiming for long term partnerships with our customers based on shared benefits and goals for synergistic growth Quality work Experienced Innovation Our Values On Time & One time delivery Mission At Chromcore, our mission is to deliver Value added, Reliable and cost effective services to our customers through our core values Good Service Professional Safety

To ensure that our services adhere to the highest standards which are on par with global quality standards and setting new benchmarks. Quality Policy To achieve sustained and profitable growth by providing services, which consistently satisfy the needs and expectations of customers We believe that quality and compliance can be designed into processes. We believe in achieving quality standards in all that we do.

Why Chromcore Lifesciences Well defined Standard Operating procedures Customer Focused Approach Customer Satisfaction is our Goal Uncompromising Commitment towards Quality Professional and experienced workforce On time delivery with quick turn around time Cost effective World Class Facility with highest quality infrastructure and instrumentation Assured data confidentiality and integrity
Chromcore Strength c. GMP compliant laboratory Chromcore is a full-service c. GMP compliant laboratory specializing in analytical testing of a range of inhaled drug delivery systems. Capabilities We provide comprehensive solutions for all the inhalation dosage forms from early stage of product development to commercialization and we can also provide support for post approval amendment filing process. Expertise Our scientists utilize the latest instrumentation and methodologies to provide customers with the highest quality of analytical research across all inhalation dosage forms( MDI’s, DPI’s, nebulizers, and nasal drug products). Global regulatory expertise Utilizing our global regulatory expertise, we will customize a program to meet your In-Vitro Bio- equivalence testing specific requirements Project Management Extensive project management will ensure sponsor involvement and a smooth transition of data and documentation
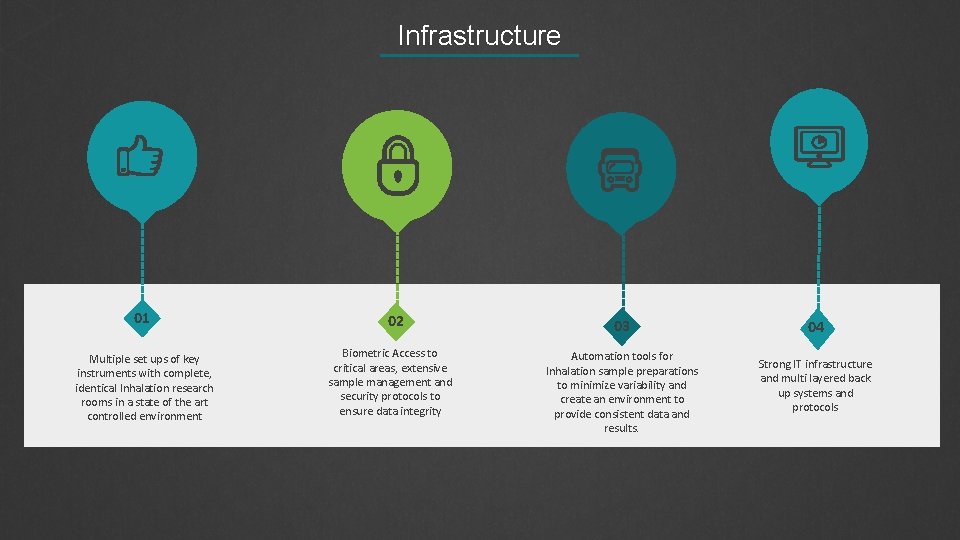
Infrastructure 01 Multiple set ups of key instruments with complete, identical Inhalation research rooms in a state of the art controlled environment 02 Biometric Access to critical areas, extensive sample management and security protocols to ensure data integrity 03 Automation tools for Inhalation sample preparations to minimize variability and create an environment to provide consistent data and results. 04 Strong IT infrastructure and multi layered back up systems and protocols

Expertize in all delivery systems Metered Dose Inhalers Dry Powder Inhalers Nasal Sprays Nebulizers

Tests For DPI’s SPECIFICATIONS FOR THE DRUG PRODUCT • Appearance and Colour DRUG PRODUCT CHARACTERIZATION STUDIES FOR SUBMISSION INNOVATIVE IN-VITRO TESTING • BRS 3000 -Improving IVIVCs for DPIs • Microbial Limit Test • Determination of Appropriate Storage Conditions • Dissolution Testing with out using anatomical throat • Stability of Primary (Unprotected) Package • Water or Moisture Content • Effect of Varying Flow Rates • Morphology • Net Content (Fill) Weight (Device-metered) • Effect of Storage on the Particle Size Distribution • Surface area • Drug Content (Assay) • Dose Build-up and Flow Resistance • Surface energy • Impurities and Degradation Products • Effect of Orientation • Powder Rheology (FT 4) • Dose Content Uniformity • In Vitro Dose Proportionality • Particle Size and shape • Dose Content Uniformity Through Container • Effect of Patient Use / In-use stability (as per PIL) • Cohesive adhesive balance • Identification (Primary and secondary) Life (device-metered) • Effect of Moisture • Particle Size Distribution of Emitted Dose • Photo stability • Microscopic Evaluation • Profiling of Doses Near Device Exhaustion • Priming ( For Device metered) • Fill Weight • Device Ruggedness • Cleaning Instructions IN-VITRO BIO- EQUIVALENCE WITH STATISTICAL ANALYSIS (FOR SUBMISSION) • APSD (test and Reference) with critical stage PBE • Single actuation content (test and Reference) (SAC) with PBE • Q 1 -Q 2 comparison
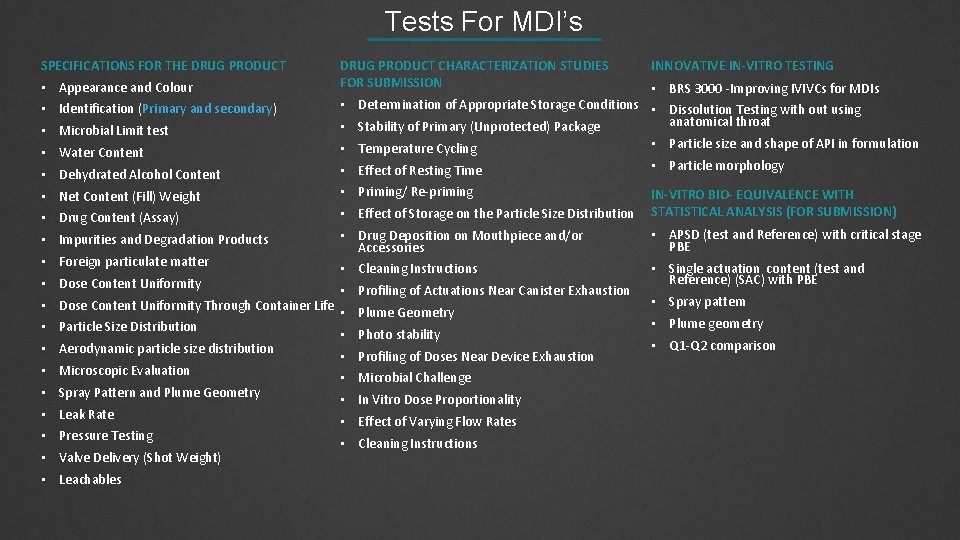
Tests For MDI’s SPECIFICATIONS FOR THE DRUG PRODUCT • Appearance and Colour DRUG PRODUCT CHARACTERIZATION STUDIES FOR SUBMISSION INNOVATIVE IN-VITRO TESTING • BRS 3000 -Improving IVIVCs for MDIs • Water Content • Determination of Appropriate Storage Conditions • Dissolution Testing with out using anatomical throat • Stability of Primary (Unprotected) Package • Particle size and shape of API in formulation • Temperature Cycling • Dehydrated Alcohol Content • Effect of Resting Time • Particle morphology • Net Content (Fill) Weight • Priming/ Re-priming • Drug Content (Assay) • Effect of Storage on the Particle Size Distribution IN-VITRO BIO- EQUIVALENCE WITH STATISTICAL ANALYSIS (FOR SUBMISSION) • Impurities and Degradation Products • Drug Deposition on Mouthpiece and/or Accessories • APSD (test and Reference) with critical stage PBE • Cleaning Instructions • Single actuation content (test and Reference) (SAC) with PBE • Identification (Primary and secondary) • Microbial Limit test • Foreign particulate matter • Dose Content Uniformity Through Container Life • Particle Size Distribution • Aerodynamic particle size distribution • Microscopic Evaluation • Spray Pattern and Plume Geometry • Leak Rate • Pressure Testing • Valve Delivery (Shot Weight) • Leachables • Profiling of Actuations Near Canister Exhaustion • Plume Geometry • Photo stability • Profiling of Doses Near Device Exhaustion • Microbial Challenge • In Vitro Dose Proportionality • Effect of Varying Flow Rates • Cleaning Instructions • Spray pattern • Plume geometry • Q 1 -Q 2 comparison

Tests For Nebulizers/ Inhalation solution and Suspension’s SPECIFICATIONS FOR THE DRUG PRODUCT CHARACTERIZATION STUDIES FOR SUBMISSION • Description • Identification (primary and secondary). • Determination of Appropriate Storage Conditions • p. H, Osmolality • Stability of Primary (Unprotected) Package • Assay • Temperature Cycling • Unit Dose Content Uniformity • • Foreign particulate matter • Concentration of tonicity agent (if any) • Limit of chelating agent (if any). • Particle size distribution of drug in immediate container. INNOVATIVE IN-VITRO TESTING • BRS 2000 -Improving IVIVCs for nebulizers • Breathing profile with face masks • Dissolution Testing with out using anatomical throat Effect of Storage on the Particle Size Distribution • Particle size and shape of API in formulation • Photo stability • Particle morphology • Droplet size distribution • Sterility IN-VITRO BIO- EQUIVALENCE WITH STATISTICAL ANALYSIS (FOR SUBMISSION) • In Vitro Dose Proportionality • Drug delivery at 60 s • APSD (test and Reference) with critical stage PBE • Polymorph form comparison • Related substances/ Degradation profile • Mean Nebulization time • Mean delivered dose • Particle size distribution • Aerodynamic particle size distribution • Particle shape • Sterility • Uniformity of dosage unit • Leachables • Droplet size distribution • Q 1 -Q 2 comparison

Tests For Nasal Sprays SPECIFICATIONS FOR THE DRUG PRODUCT CHARACTERIZATION STUDIES FOR SUBMISSION INNOVATIVE IN-VITRO TESTING • Description • Identification (primary and secondary). • Determination of Appropriate Storage Conditions • p. H, Osmolality • Stability of Primary (Unprotected) Package • Valve Delivery (Shot Weight) • Temperature Cycling • Assay • Effect of Storage IN-VITRO BIO- EQUIVALENCE WITH STATISTICAL ANALYSIS (FOR SUBMISSION) • Unit Dose Content Uniformity • Droplet size distribution • • Foreign particulate matter • Plume Geometry Single actuation content (test and Reference) (SAC) with PBE Photo stability • Droplet size distribution Concentration of tonicity agent (if any) • Drug in small particle/ droplet Limit of chelating agent (if any). Aerodynamic particle size distribution with suitable expansion chamber • • Spray pattern • Droplet size distribution • Plume Geometry • Spray pattern and Plume geometry • Priming and Re-priming • Related substances/ Degradation profile • Q 1 -Q 2 comparison • Sterility • Leachables • • Sterility • Particle size and shape of API in formulation • Particle morphology
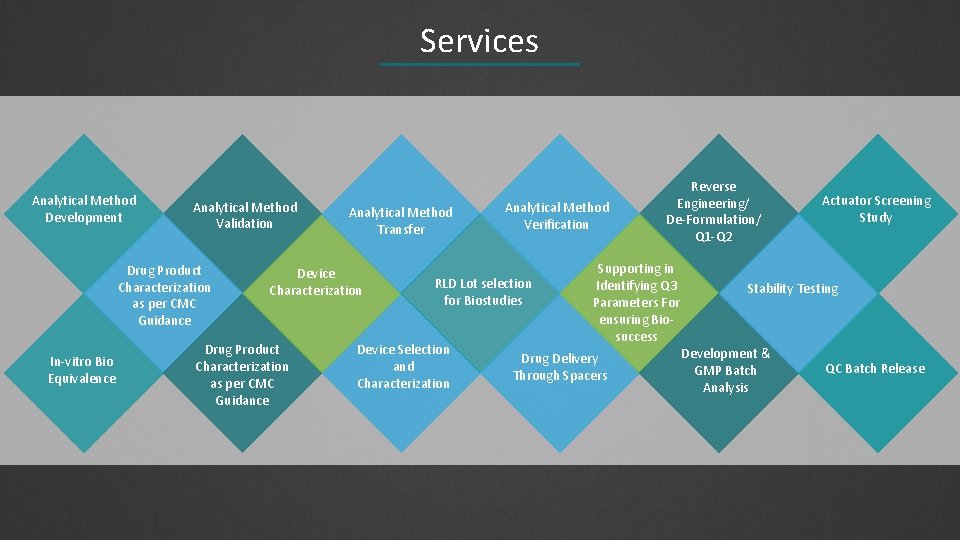
Services Analytical Method Development Analytical Method Validation Drug Product Characterization as per CMC Guidance In-vitro Bio Equivalence Analytical Method Transfer Device Characterization Drug Product Characterization as per CMC Guidance Analytical Method Verification RLD Lot selection for Biostudies Device Selection and Characterization Reverse Engineering/ De-Formulation/ Q 1 -Q 2 Supporting in Identifying Q 3 Parameters For ensuring Biosuccess Drug Delivery Through Spacers Actuator Screening Study Stability Testing Development & GMP Batch Analysis QC Batch Release
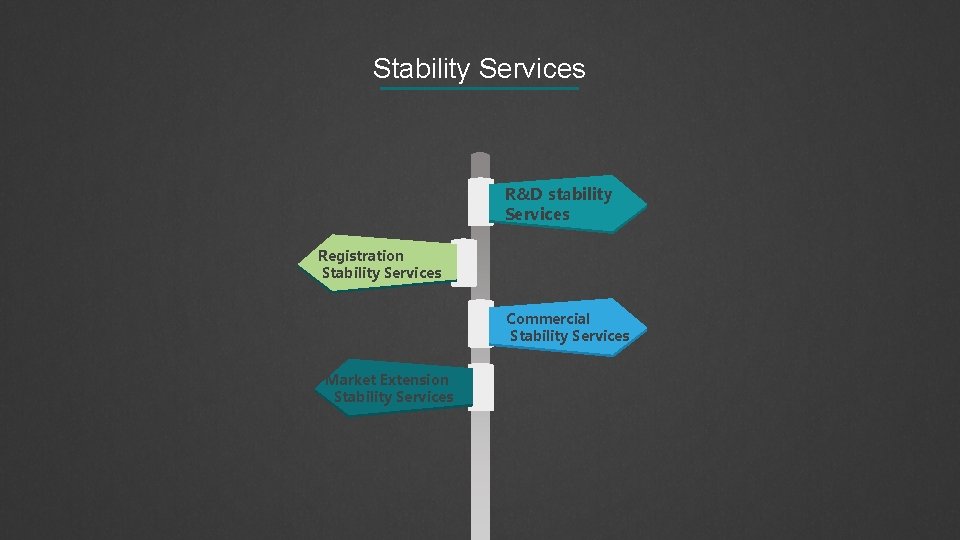
Stability Services R&D stability Services Registration Stability Services Commercial Stability Services Market Extension Stability Services
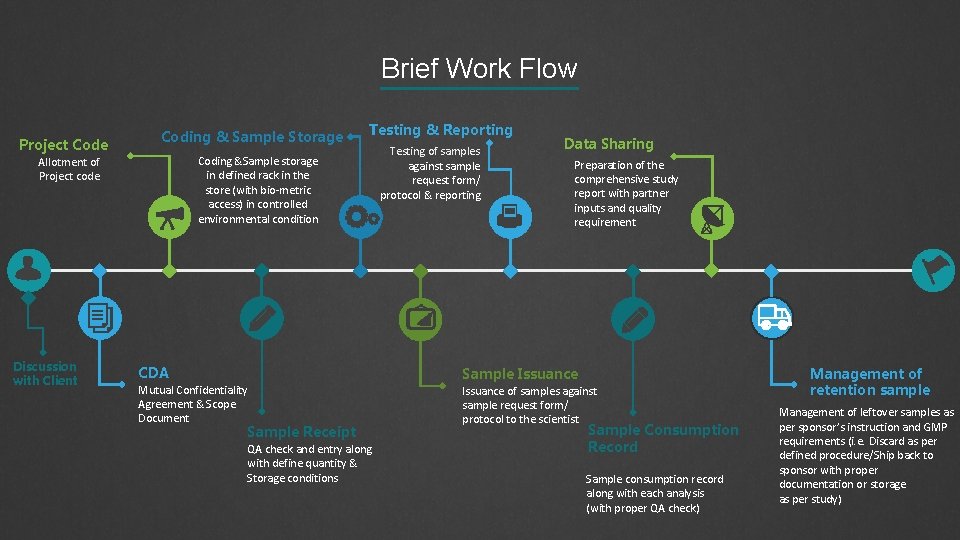
Brief Work Flow Project Code Coding & Sample Storage Coding &Sample storage in defined rack in the store (with bio-metric access) in controlled environmental condition Allotment of Project code Discussion with Client Testing & Reporting CDA Mutual Confidentiality Agreement & Scope Document Sample Receipt QA check and entry along with define quantity & Storage conditions Testing of samples against sample request form/ protocol & reporting Data Sharing Preparation of the comprehensive study report with partner inputs and quality requirement Sample Issuance of samples against sample request form/ protocol to the scientist Sample Consumption Record Sample consumption record along with each analysis (with proper QA check) Management of retention sample Management of leftover samples as per sponsor’s instruction and GMP requirements (i. e. Discard as per defined procedure/Ship back to sponsor with proper documentation or storage as per study)
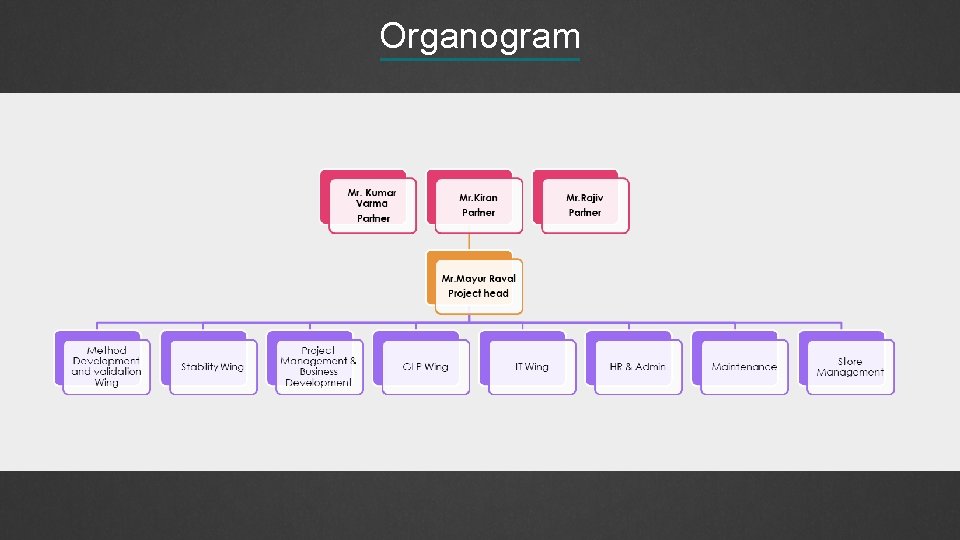
Organogram

Instrumentation List Wet Lab Sr. No Instrument Name Make/Model No. 1 p. H meter Thermo 2 Ultra micro balance Range: 2 g / 0. 1 μg Radwag UYA 2. 4 Y 3 Semi micro balance Range: 82/220 g - 0. 01 / 0. 1 mg Radwag XA 82/220. 4 Y 4 Precision balance Range: 600 g / 1 mg Radwag PS 600. X 2 5 KF Coulometer Metrohm 6 Ultrasonic Bath iultrasonic 7 Refrigerator Thermolabs 8 Hot air Oven Thermolabs 9 Water Bath with temp controller iultrasonic 10 Water purification system Elga purelab option Q 7 11 Fume Hood KEMI

Instrumentation List Inhalation Lab Sr. No 1 Instrument Name Dosage Unit Sampling Apparatus (DUSA) for MDIs 2 Dosage Unit Sampling Apparatus (DUSA) for DPIs 3 Waste Shot Collector WSC 2 4 Make/Model No. Facemask Test Apparatus for Spacers & VHCs Model FMA ( Adult , Child & Infants Models) 5 Andersen Cascade Impactor 6 Next Generation Impactor 7 Glass Twin Impinger 8 Expansion Chambers (1000, 2000, 5000 lts) 9 NGI Cooler 10 Fast Screening Impactor 11 Breath Simulator BRS 2000 12 Critical Flow Controller Model TPK 2000 13 Flow Meters 14 Super Capacity Pump Model SCP 5 15 High Capacity Pump Model HCP Copley Scientific

Instrumentation List Inhalation Lab Sr. No Instrument Name 16 Dissolution Testing - Dissolution apparatus & NGI Dissolution Cup and Membrane Holder 17 ALBERTA IDEALISED THROAT 18 Apparatus for Testing Fluticasone Propionate/Salmeterol DPI's & MDI's 19 Labour Saving Tools - Delivered Dose Uniformity Make/Model No. Copley Scientific 20 Labour Saving Tools - Sample Preparation (ACI & NGI) 21 Labour Saving Tools - Cup Coating (NGI) 22 Antistatic Grounding Kit 23 Electrostatic Eliminator 24 Digital Static Meter 25 Copley Inhaler Testing Data Analysis Software (CITDAS) 26 Light Microscope with 10 X eye piece and 20 X Objective Nikon microscope & Image Provision Software 27 Laminar Air Flow Cabinet Aitech 28 FT 4 Powder Rheometer Freeman technology 29 Freezer : -86°C Arctiko ULUF 65
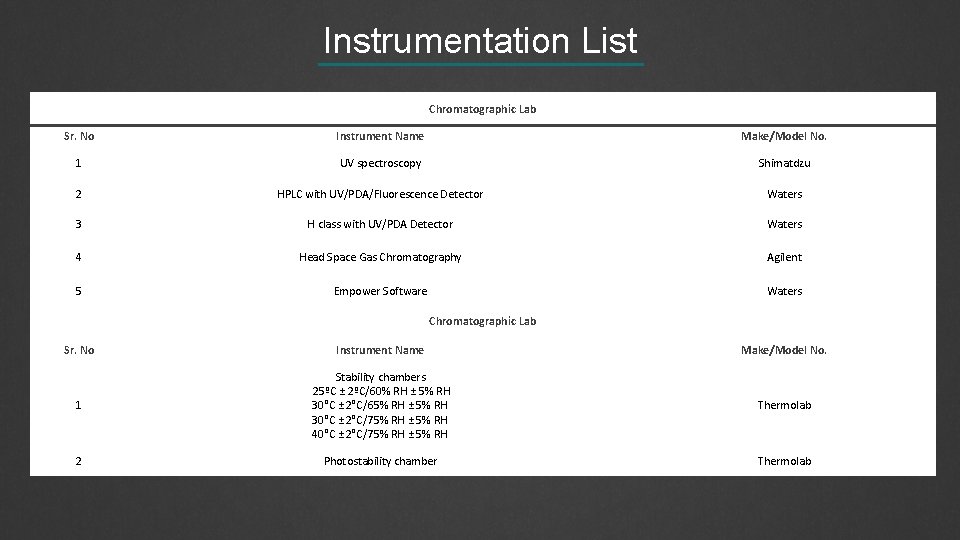
Instrumentation List Chromatographic Lab Sr. No Instrument Name Make/Model No. 1 UV spectroscopy Shimatdzu 2 HPLC with UV/PDA/Fluorescence Detector Waters 3 H class with UV/PDA Detector Waters 4 Head Space Gas Chromatography Agilent 5 Empower Software Waters Chromatographic Lab Sr. No Instrument Name Make/Model No. 1 Stability chambers 25ºC ± 2ºC/60% RH ± 5% RH 30°C ± 2°C/65% RH ± 5% RH 30°C ± 2°C/75% RH ± 5% RH 40°C ± 2°C/75% RH ± 5% RH Thermolab 2 Photostability chamber Thermolab

State of Art Infrastructure

For further enquiries Contact Us Address Cynosure plaza , 3 th floor, Madhuranagar, Visakhapatnam 530016 Phone +91 -9881499920 E-mail office@Chromcore. com Website www. chromcore. com Contact us to arrange a visit to our lab
- Slides: 21