PROGRESS INDICATORS Good progress Outstanding progress Grade 4






- Slides: 13

PROGRESS INDICATORS Good progress Outstanding progress Grade 4 -6 Define what a salt is and describe how to make a salt by reacting a metal with an acid. Write a word and balanced symbol equation to describe a reaction between a metal and sulfuric acid or hydrochloric acid. Grade 7 -9 Write a word and balanced symbol equation to describe a reaction between a metal and sulfuric acid or hydrochloric acid. Write ionic and half equations, including state symbols, to describe a reaction between a metal and sulfuric acid or hydrochloric acid. 25 May 2021

ACTIVITY 1 - DEFINE WHAT A SALT IS 1. What is the chemistry definition of salt? 2. Which groups usually give the cation for the salt? 3. Which groups usually give the anion for the salt? https: //www. youtube. com/watch? v=Wn AKhtn. Jjz 0 Watch Video to 2: 27 4. List 2 examples of salts 5. List 2 uses of salts

ACTIVITY 1 - ANSWERS 1. A salt is an ionic compound that is electrically neutral 2. Group 1 (Alkali metals) and Group 2 3. Group 7 (Halides) https: //www. youtube. com/watch? v=Wn AKhtn. Jjz 0 SA In red pens please 4. Sodium Chloride (Table salt), Sodium Chromate, Potassium Permanganate, Iron disulfide (fools gold), lead diacetate 5. Dyes for clothes and

25 May 2021 WORD CONSCIOUSNESS Salt - A salt is an ionic compound that is electrically neutral Excess - a chemical that is added into a reaction with a greater amount than necessary to react completely therefore does not dissolve. Effervescence - Fizzing/ bubbling

HOW TO REACT A METAL AND ACID 1. Zinc was first added to the acid. – Fizzing/bubbles/ Gas (hydrogen) being produced Excess zinc was added. - Powder left at the bottom that does not dissolve/stir in The salt was left to crystallise. -Crystals form on the edges of the evaporating basin, liquid evaporates 2. Create a flow chart of the steps needed to make zinc SA In red pens sulfate 25 May 2021

ACTIVITY 3 - WRITE A WORD AND BALANCED SYMBOL EQUATION TO DESCRIBE A REACTION BETWEEN A METAL AND SULFURIC ACID OR HYDROCHLORIC ACID. Complete the questions below 1. Write the word equation for the reaction between Zinc and sulfuric acid 2. a. Complete the symbol equation for this reaction ______ + H 2 SO 4 Zn. SO 4 + _______ b. Add state symbols to the equation 3. Complete the word equations for the following reactions a. Zinc +Hydrochloric acid b. Iron + Sulfric Acid 4. Complete and balance the symbol equations for the following reactions: a. ____ + HCl Fe. Cl 2 + ____ b. Mg + _______ Mg. SO 4 + ______

ACTIVITY 3 – ANSWERS Complete the questions below 1. Zinc + Sulfuric Acid Zinc Sulfate + Hydrogen 2. a. & b. Zn (s)+ H 2 SO 4 (aq) Zn. SO 4 (s) + H 2 (g) 3. a. Zinc +Hydrochloric acid Zinc Chloride + Hydogen b. Iron + Sulfric Acid Iron Sulfate + Hydrogen 4. a. Fe + 2 HCl Fe. Cl 2 + H 2 b. Mg + H 2 SO 4 Mg. SO 4 + H 2 SA In red pens please

HT ONLY

25 May 2021 WORD CONSCIOUSNESS – HT ONLY Oxidation – Loss of electrons (OILRIG) Reduction - Gain of electrons (OILRIG) Half Equation – An chemical equation that is split to look at what is happening in terms of electrons

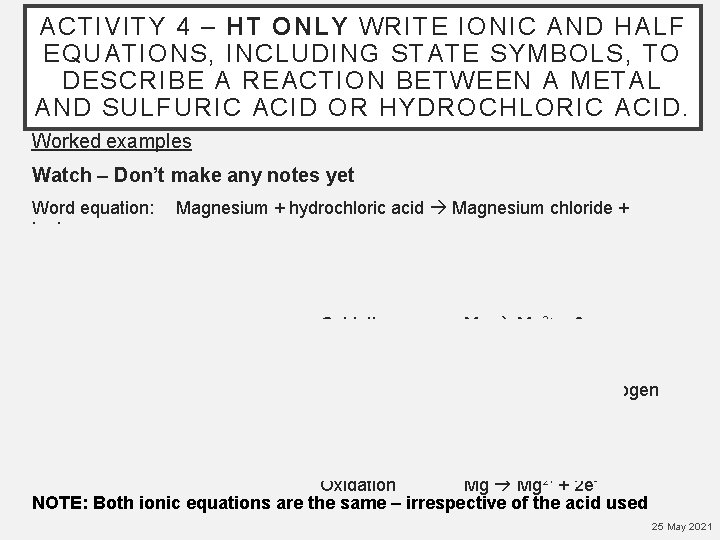
ACTIVITY 4 – HT ONLY WRITE IONIC AND HALF EQUATIONS, INCLUDING STATE SYMBOLS, TO DESCRIBE A REACTION BETWEEN A METAL AND SULFURIC ACID OR HYDROCHLORIC ACID. Worked examples Watch – Don’t make any notes yet Word equation: hydrogen Magnesium + hydrochloric acid Magnesium chloride + Balanced symbol equation: Ionic equations: Mg + 2 HCl Mg. Cl 2 + H 2 Reduction 2 H+ + 2 e- H 2 Oxidation Mg 2+ + 2 e- Together now! Tell me what to do! Word equation: Magnesium + sulfuric acid Magnesium sulfate + hydrogen Balanced symbol equation: Ionic equations: Mg + H 2 SO 4 Mg. SO 4 + H 2 Reduction 2 H+ + 2 e- H 2 Oxidation Mg 2+ + 2 e. NOTE: Both ionic equations are the same – irrespective of the acid used 25 May 2021

ACTIVITY 4 – HT ONLY WRITE IONIC AND HALF EQUATIONS, INCLUDING STATE SYMBOLS, TO DESCRIBE A REACTION BETWEEN A METAL AND SULFURIC ACID OR HYDROCHLORIC ACID. Your turn! Have a go at working out the ionic half equations for these reactions 1. Potassium + hydrochloric acid Potassium chloride + hydrogen 2. Calcium + Sulfuric acid Calcium Sulfate + Hydrogen 3. Potassium + Sulfuric acid Potassium sulfate + hydrogen 4. Sodium + Hydrochloric acid Sodium Chloride + Hydrogen 25 May 2021

ACTIVITY 4 – HT ONLY ANSWERS 1. Potassium + hydrochloric acid Potassium chloride + hydrogen 2 K + 2 HCl 2 KCl +H 2 Oxidation = 2 K 2 K+ + 2 e- Reduction = 2 H+ + 2 e- H 2 2. Calcium + Sulfuric acid Calcium Sulfate + Hydrogen Ca + H 2 SO 4 Ca. SO 4 +H 2 Oxidation = Ca 2+ + 2 e- Reduction = 2 H+ + 2 e- H 2 3. Potassium + Sulfuric acid Potassium sulfate + hydrogen 2 K + H 2 SO 4 K 2 SO 4 + H 2 Oxidation = 2 K 2 K+ + 2 e- Reduction = 2 H+ + 2 e- H 2 4. Sodium + Hydrochloric acid Sodium Chloride + Hydrogen 2 Na + 2 HCl 2 Na. Cl +H 2 Oxidation = 2 Na+ + 2 e- Reduction = 2 H+ + 2 e- H 2 SA In red pens please 25 May 2021

PLENARY: POST IT NOTE CAR PARK Write one fact that you have learnt and one question you are still unsure about. Post it on the car park!