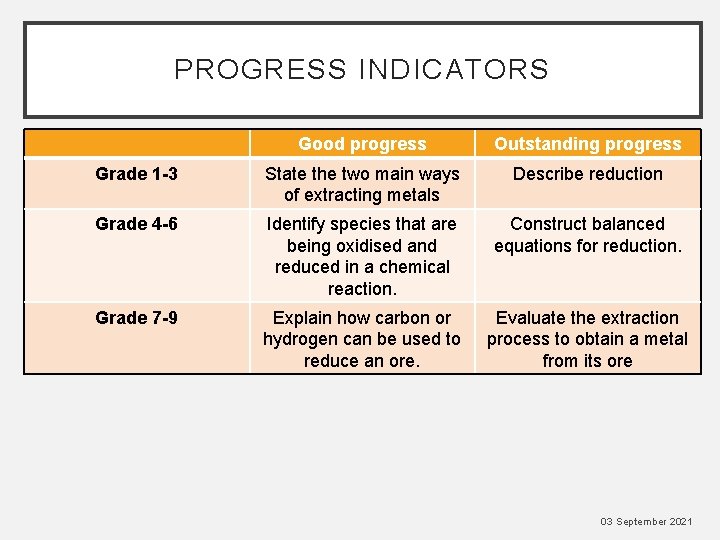
PROGRESS INDICATORS Good progress Outstanding progress Grade 1



- Slides: 11

PROGRESS INDICATORS Good progress Outstanding progress Grade 1 -3 State the two main ways of extracting metals Describe reduction Grade 4 -6 Identify species that are being oxidised and reduced in a chemical reaction. Construct balanced equations for reduction. Grade 7 -9 Explain how carbon or hydrogen can be used to reduce an ore. Evaluate the extraction process to obtain a metal from its ore 03 September 2021

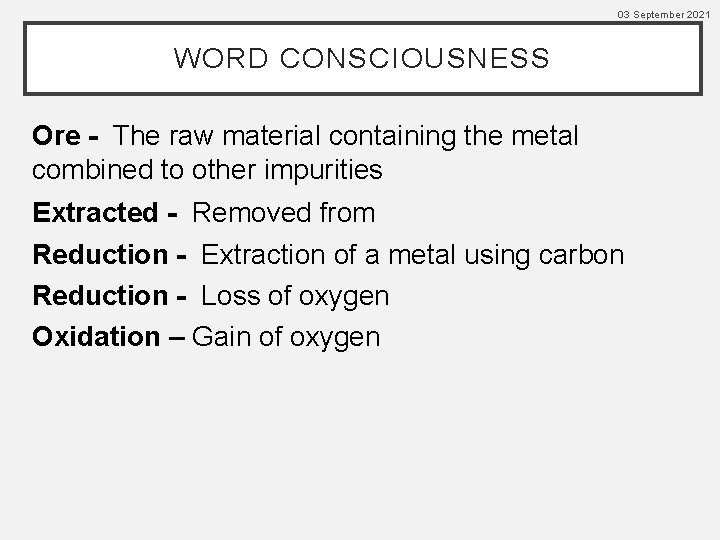
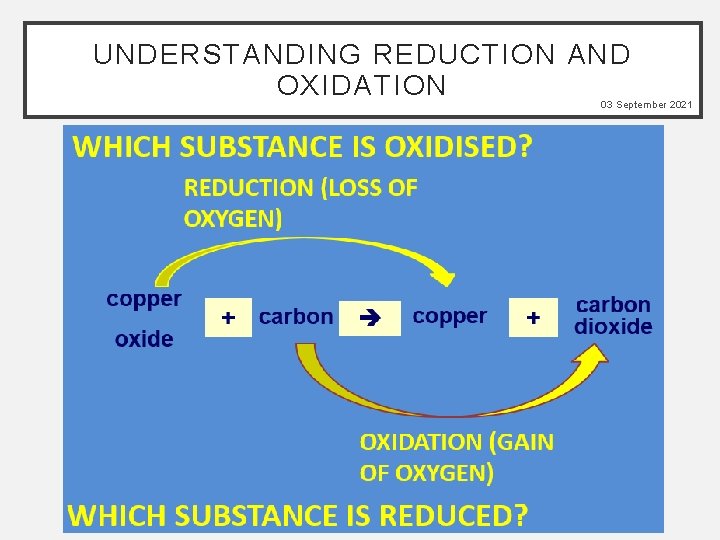
03 September 2021 WORD CONSCIOUSNESS Ore - The raw material containing the metal combined to other impurities Extracted - Removed from Reduction - Extraction of a metal using carbon Reduction - Loss of oxygen Oxidation – Gain of oxygen

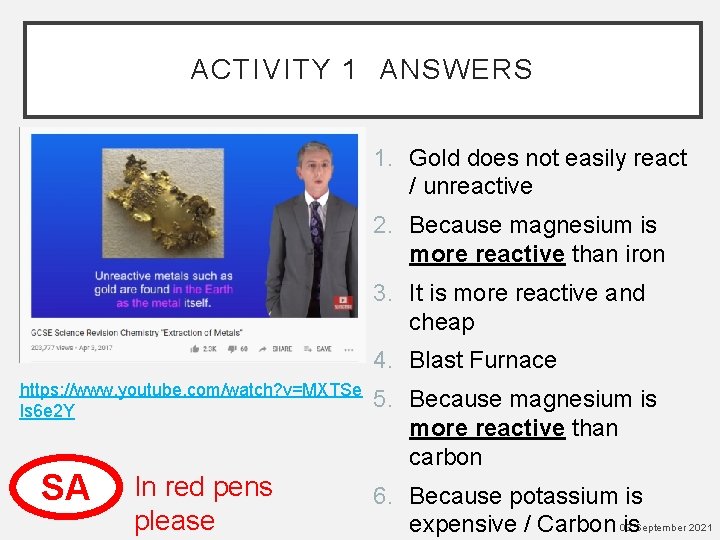
ACTIVITY 1 - DESCRIBE THE TWO MAIN METHODS OF EXTRACTION 1. Why is gold found in the Earth as a metal itself? 2. Why can magnesium displace iron in iron oxide? 3. Why do we use carbon to extract iron? 4. What is the process of extracting a metal using https: //www. youtube. com/watch? v=MXTSe carbon called? ls 6 e 2 Y 5. Why can we not use carbon to extract magnesium? 6. Why do we not use

ACTIVITY 1 ANSWERS 1. Gold does not easily react / unreactive 2. Because magnesium is more reactive than iron 3. It is more reactive and cheap 4. Blast Furnace https: //www. youtube. com/watch? v=MXTSe ls 6 e 2 Y SA In red pens please 5. Because magnesium is more reactive than carbon 6. Because potassium is expensive / Carbon 03 is. September 2021


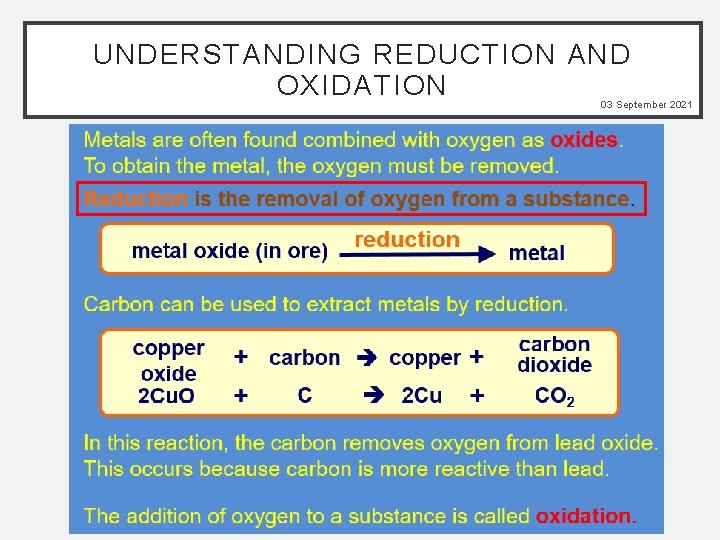
UNDERSTANDING REDUCTION AND OXIDATION 03 September 2021

UNDERSTANDING REDUCTION AND OXIDATION 03 September 2021

Record it! You have 3 minutes to record what you can remember – by yourself You will then have 3 minutes to work with your shoulder buddy to share and check you are not missing any points

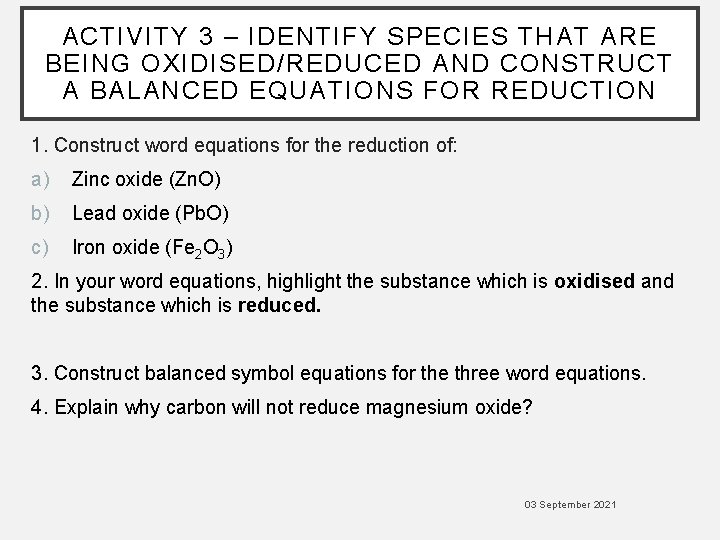
ACTIVITY 3 – IDENTIFY SPECIES THAT ARE BEING OXIDISED/REDUCED AND CONSTRUCT A BALANCED EQUATIONS FOR REDUCTION 1. Construct word equations for the reduction of: a) Zinc oxide (Zn. O) b) Lead oxide (Pb. O) c) Iron oxide (Fe 2 O 3) 2. In your word equations, highlight the substance which is oxidised and the substance which is reduced. 3. Construct balanced symbol equations for the three word equations. 4. Explain why carbon will not reduce magnesium oxide? 03 September 2021

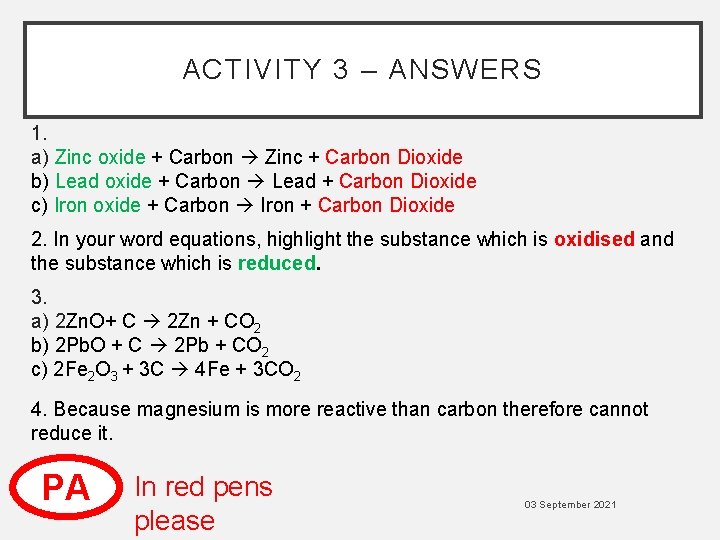
ACTIVITY 3 – ANSWERS 1. a) Zinc oxide + Carbon Zinc + Carbon Dioxide b) Lead oxide + Carbon Lead + Carbon Dioxide c) Iron oxide + Carbon Iron + Carbon Dioxide 2. In your word equations, highlight the substance which is oxidised and the substance which is reduced. 3. a) 2 Zn. O+ C 2 Zn + CO 2 b) 2 Pb. O + C 2 Pb + CO 2 c) 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2 4. Because magnesium is more reactive than carbon therefore cannot reduce it. PA In red pens please 03 September 2021

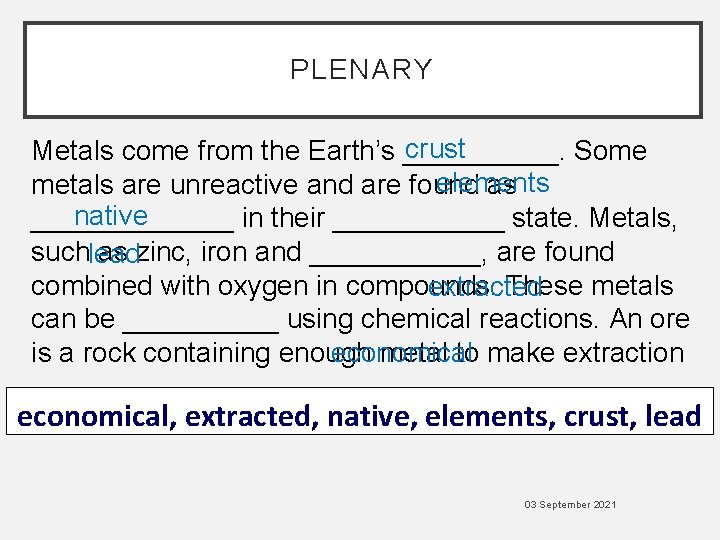
PLENARY crust Metals come from the Earth’s _____. Some elements metals are unreactive and are found as native _______ in their ______ state. Metals, suchlead as zinc, iron and ______, are found combined with oxygen in compounds. These metals extracted can be _____ using chemical reactions. An ore is a rock containing enough metal to make extraction economical ______. economical, extracted, native, elements, crust, lead 03 September 2021