Progress in the HIV Vaccine Field Larry Corey




























- Slides: 61

Progress in the HIV Vaccine Field Larry Corey, MD Principal Investigator, NIAID supported HIV Vaccine Trials Network (HVTN) Past President and Director, Fred Hutchinson Cancer Research Center Professor, Laboratory Medicine and Medicine, University of Washington Seattle, Washington USA

Acknowledgements • HVTN as an organization, especially my colleagues: Scott Hammer, Glenda Gray, Julie Mc. Elrath, Peter Gilbert, Jim Kublin and Susan Buchbinder • The HVTN’s major pharmaceutical and institutional collaborators: Sanofi, GSK, Janssen, VRC, IAVI and CHAVI programs • The HVTN’s community members and advisory boards • Its funders: NIAID and BMGF

Special Acknowledgement Tony Fauci, MD

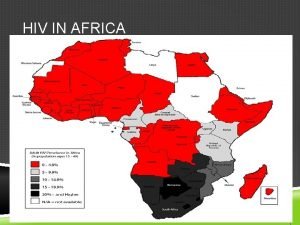
HIV: Still the World’s Most Important Global Health Problem • US still over 45, 000 new cases yearly • Globally more than 2 million new infections occur per year • Number of people living with HIV increasing yearly • Long way from an AIDS Free Generation

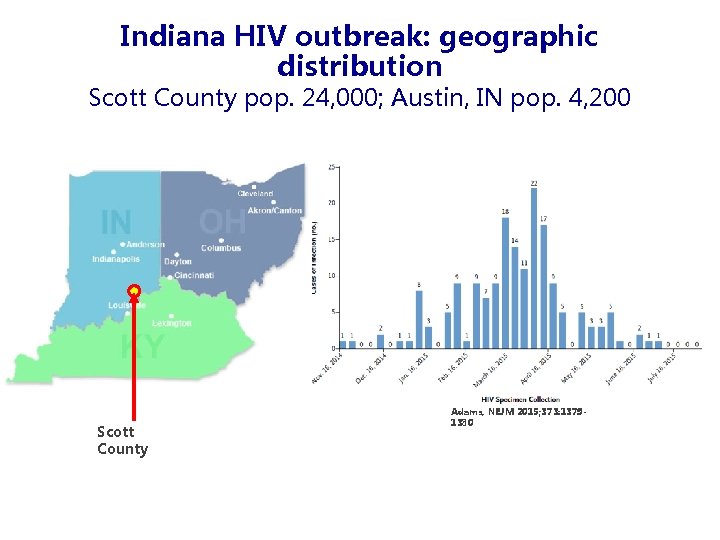
Indiana HIV outbreak: geographic distribution Scott County pop. 24, 000; Austin, IN pop. 4, 200 Scott County Adams, NEJM 2015; 373: 13791380


Commentary on HIV Prevention Strategies • While many prevention strategies have high efficacy in clinical trials, their extended effectiveness requires continuous adherence, which often results in decreased effectiveness over time. • They also require high saturation in a community and hence their long term effects on population based incidence in country’s with generalized epidemics is uncertain: • Condoms; Pr. EP; vaginal rings; PEP; circumcision, all deserve support and increased uptake • Test and Treat very effective for the individual; eventual population effect will be achieved

The Need for an HIV Vaccine • With asymptomatic acquisition, prolonged subclinical infection, and sexual transmission, getting to an AIDS Free Generation will require a vaccine. • Larry’s definition of an AIDS Free Generation; 95% reduction in incident cases annually: • USA < 2, 500 incident cases yearly • Globally < 100, 000 cases yearly 9/25/2020 9

“Ultimately, we believe, the only guarantee of a sustained end of th AIDS pandemic lies in a combination of non-vaccine prevention methods and the development and deployment of a safe and effective HIV vaccine. ”

The Two Major Scientific Questions Facing the HIV Vaccine Field: • Can non-neutralizing antibodies be potent enough to achieve desirable vaccine efficacy (VE >50%) for at least 2 years? • Can this be achieved by designing better recombinant proteins and adjuvants? • By eliciting better T helper responses to drive higher and more durable antibody production? • Is neutralization, as we currently measure it, associated with vaccine protection and will this protection be of a sufficient magnitude to overshadow other vaccine design approaches?

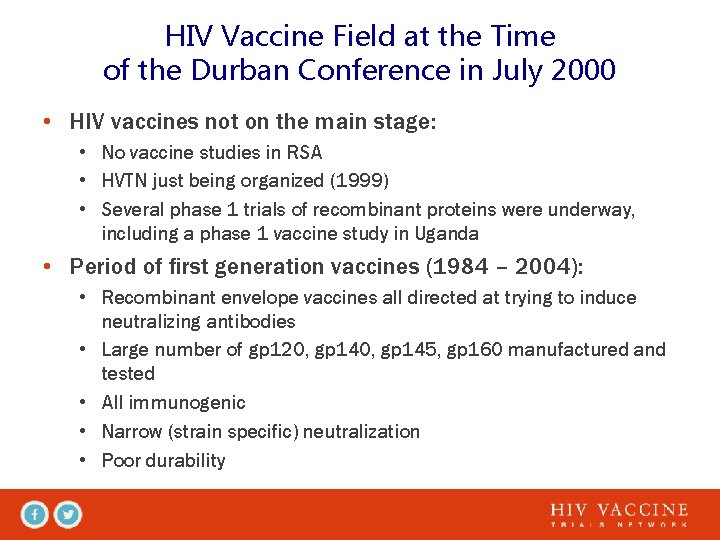
HIV Vaccine Field at the Time of the Durban Conference in July 2000 • HIV vaccines not on the main stage: • No vaccine studies in RSA • HVTN just being organized (1999) • Several phase 1 trials of recombinant proteins were underway, including a phase 1 vaccine study in Uganda • Period of first generation vaccines (1984 – 2004): • Recombinant envelope vaccines all directed at trying to induce neutralizing antibodies • Large number of gp 120, gp 145, gp 160 manufactured and tested • All immunogenic • Narrow (strain specific) neutralization • Poor durability

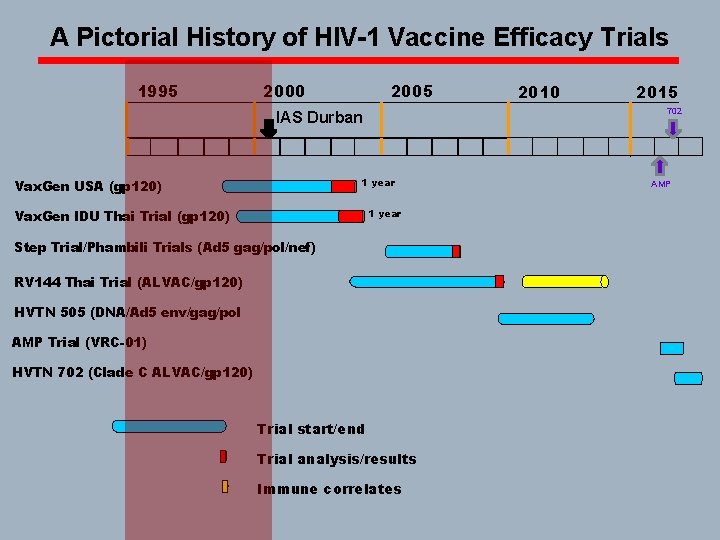
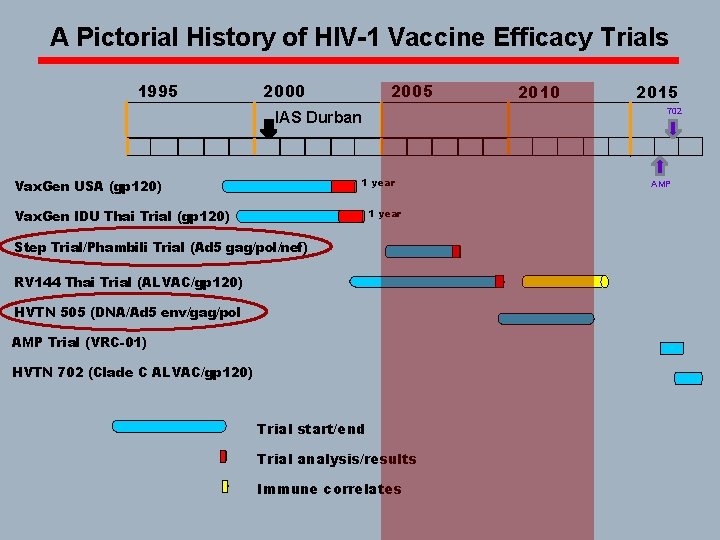
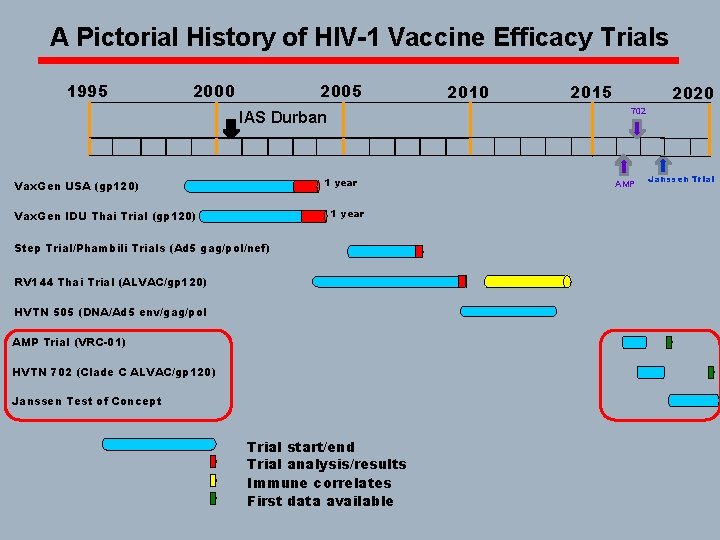
A Pictorial History of HIV-1 Vaccine Efficacy Trials 1995 2000 2005 1 year Vax. Gen IDU Thai Trial (gp 120) 2015 702 IAS Durban Vax. Gen USA (gp 120) 2010 Step Trial/Phambili Trials (Ad 5 gag/pol/nef) RV 144 Thai Trial (ALVAC/gp 120) HVTN 505 (DNA/Ad 5 env/gag/pol AMP Trial (VRC-01) HVTN 702 (Clade C ALVAC/gp 120) Trial start/end Trial analysis/results Immune correlates AMP

Second Generation Vaccines: T Cell Based Vaccines • Post-Vax. Gen, HIV vaccine field turned to “T cell based” vaccines • CD 8+ T cells were what differentiated elite controllers from progression and it was hoped that vaccines that would induce such responses would be effective in either reducing acquisition or postacquisition viral load. • Hypothesis: the more potent T cell responses, the better the vaccine: • Ad 5 vector based vaccine much more effective in inducing CD 8+ T cell responses than ALVAC

A Pictorial History of HIV-1 Vaccine Efficacy Trials 1995 2000 2005 1 year Vax. Gen IDU Thai Trial (gp 120) 2015 702 IAS Durban Vax. Gen USA (gp 120) 2010 Step Trial/Phambili Trial (Ad 5 gag/pol/nef) RV 144 Thai Trial (ALVAC/gp 120) HVTN 505 (DNA/Ad 5 env/gag/pol AMP Trial (VRC-01) HVTN 702 (Clade C ALVAC/gp 120) Trial start/end Trial analysis/results Immune correlates AMP

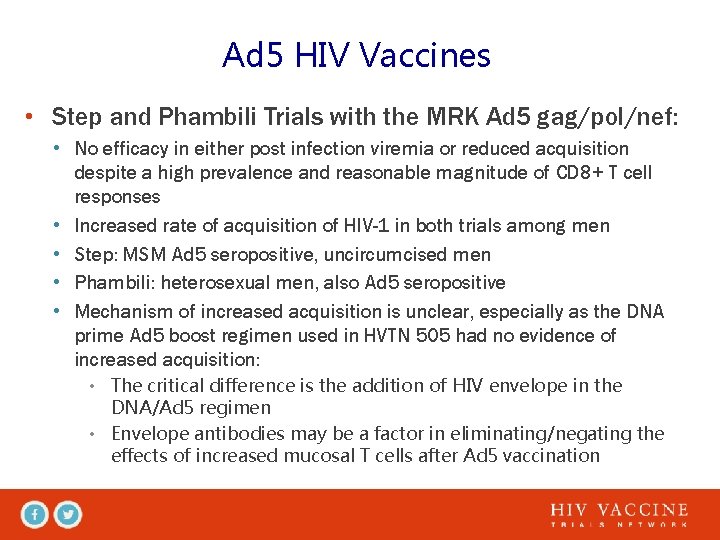
Ad 5 HIV Vaccines • Step and Phambili Trials with the MRK Ad 5 gag/pol/nef: • No efficacy in either post infection viremia or reduced acquisition despite a high prevalence and reasonable magnitude of CD 8+ T cell responses • Increased rate of acquisition of HIV-1 in both trials among men • Step: MSM Ad 5 seropositive, uncircumcised men • Phambili: heterosexual men, also Ad 5 seropositive • Mechanism of increased acquisition is unclear, especially as the DNA prime Ad 5 boost regimen used in HVTN 505 had no evidence of increased acquisition: • The critical difference is the addition of HIV envelope in the DNA/Ad 5 regimen • Envelope antibodies may be a factor in eliminating/negating the effects of increased mucosal T cells after Ad 5 vaccination

The Good News for HIV Vaccine Development – September 2009 and the RV 144 Trial • Regimen of ALVAC priming followed by gp 120 results in efficacy in large trial in Thailand. • Results met with surprise and skepticism: • ALVAC not as good as Ad vectors for T cell priming • gp 120 used had failed in IDU trial • How could these two together all of a sudden produce efficacy?

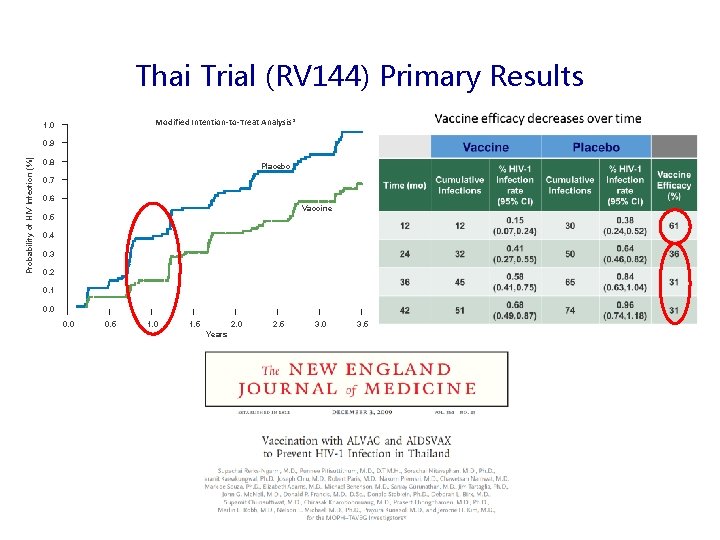
Thai Trial (RV 144) Primary Results Modified Intention-to-Treat Analysis* 1. 0 Probability of HIV Infection (%) 0. 9 0. 8 Placebo 0. 7 0. 6 Vaccine 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0. 5 1. 0 1. 5 2. 0 Years 2. 5 3. 0 3. 5

Post-RV 144 • Massive scientific effort to understand how did RV 144 work: correlates of risk/correlates of protection. • Pivot in the field from concentrating on novel vectors to understanding that it is the insert (HIV envelope gene ) and structure of the envelope antigen that one puts in the vector that is critical for vaccine design.

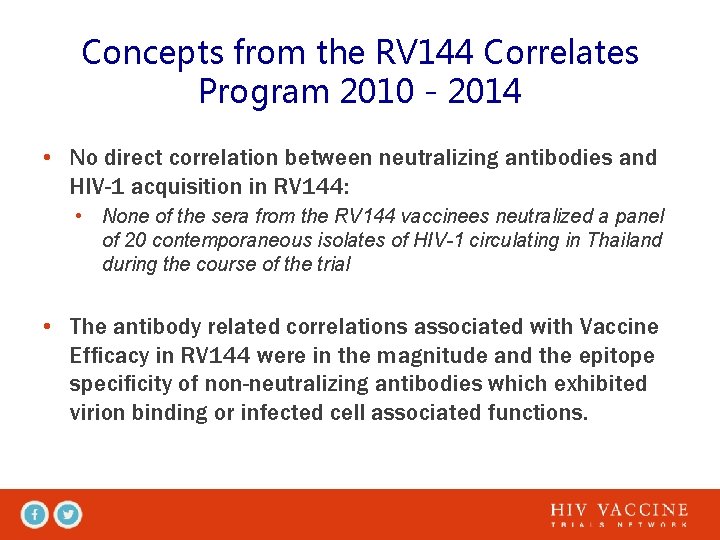
Concepts from the RV 144 Correlates Program 2010 - 2014 • No direct correlation between neutralizing antibodies and HIV-1 acquisition in RV 144: • None of the sera from the RV 144 vaccinees neutralized a panel of 20 contemporaneous isolates of HIV-1 circulating in Thailand during the course of the trial • The antibody related correlations associated with Vaccine Efficacy in RV 144 were in the magnitude and the epitope specificity of non-neutralizing antibodies which exhibited virion binding or infected cell associated functions.

Correlation Between Antibodies to the V 1 V 2 Loop and Vaccine Efficacy in RV 144 • Antibodies to the conserved region of V 2, previously almost completely ignored by the HIV vaccine field, were highly correlated with efficacy.

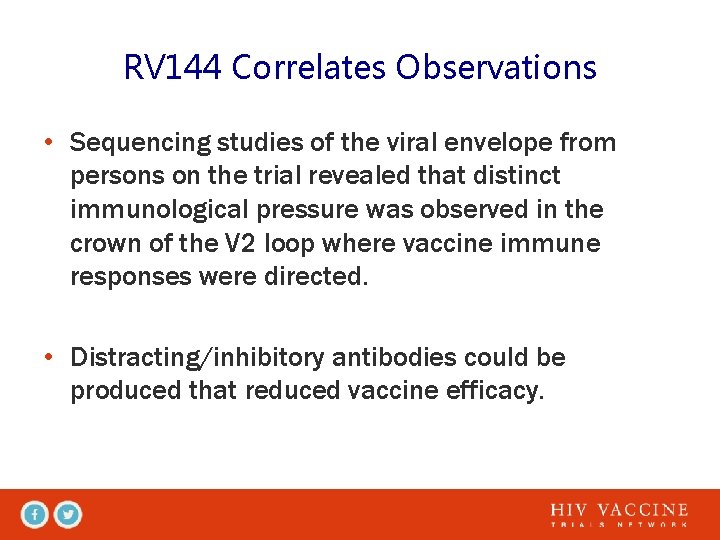
RV 144 Correlates Observations • Sequencing studies of the viral envelope from persons on the trial revealed that distinct immunological pressure was observed in the crown of the V 2 loop where vaccine immune responses were directed. • Distracting/inhibitory antibodies could be produced that reduced vaccine efficacy.

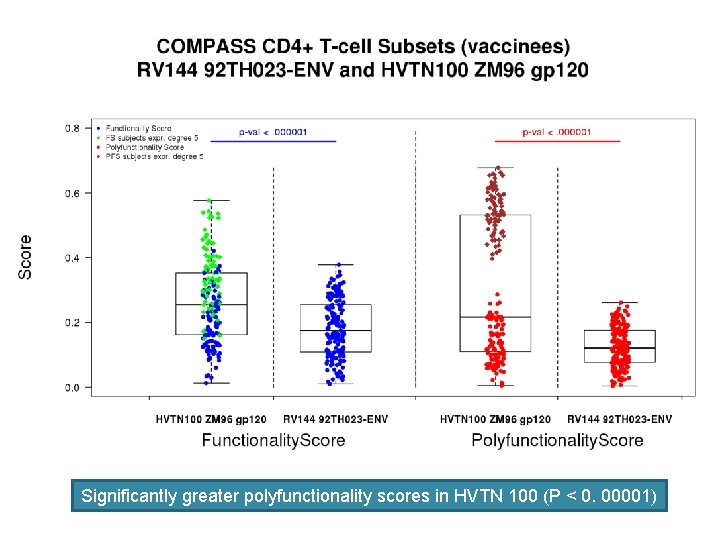
CD 4+ T cell Responses to HIV envelope in RV 144 • Polyfunctional CD 4+ T cell responses to HIV-1 envelope also independently contributed to VE. • The cytokine patterns of these helper T cell responses suggest that T helper responses that influence antibody development are important.

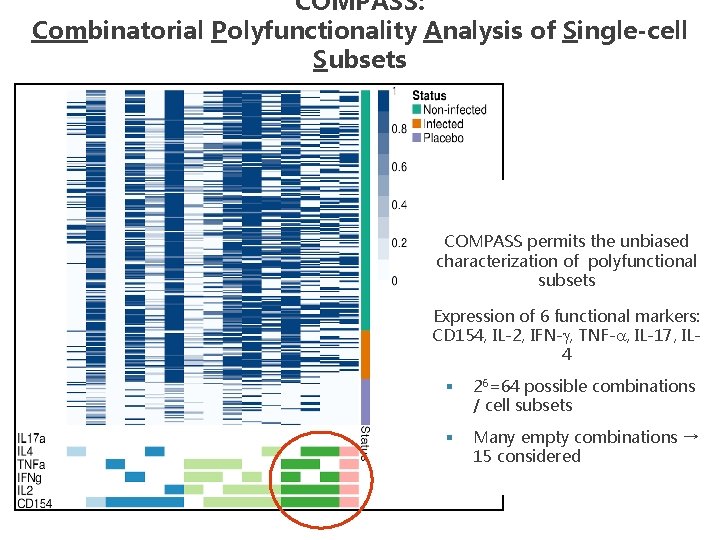
COMPASS: Combinatorial Polyfunctionality Analysis of Single-cell Subsets COMPASS permits the unbiased characterization of polyfunctional subsets Expression of 6 functional markers: CD 154, IL-2, IFN-g, TNF-a, IL-17, IL 4 § 26=64 possible combinations / cell subsets § Many empty combinations → 15 considered

Env-specific CD 4+ T cell polyfunctionality score is an independent correlate of Vaccine Efficacy The five-function CD 4+ T subset remains significant (OR=0. 59, p=0. 010) when the V 1 V 2 and Ig. A Ab variables are also included in the model. The effects of the V 1 V 2 Ig. G correlate and the five-function subset are additive (no evidence of interaction). Variable OR 95% CI P-value 5 -function Env-specific CD 4+ T cell subset 0. 59 0. 40 -0. 89 0. 011 V 1 V 2 Ig. G Ab primary 0. 62 0. 42 -0. 94 0. 022 Ig. A Ab primary 1. 76 1. 2 -2. 6 0. 003 (Lin L. et al, Nat Biotech. 2015 Jun; 33(6): 610 -6. )

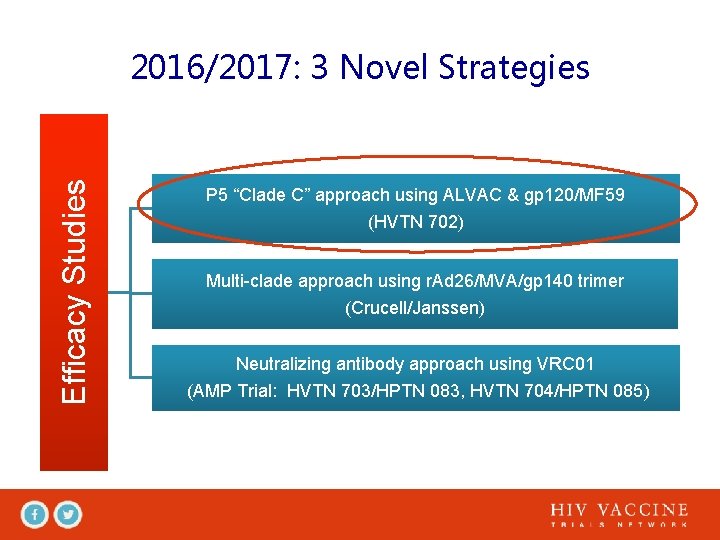
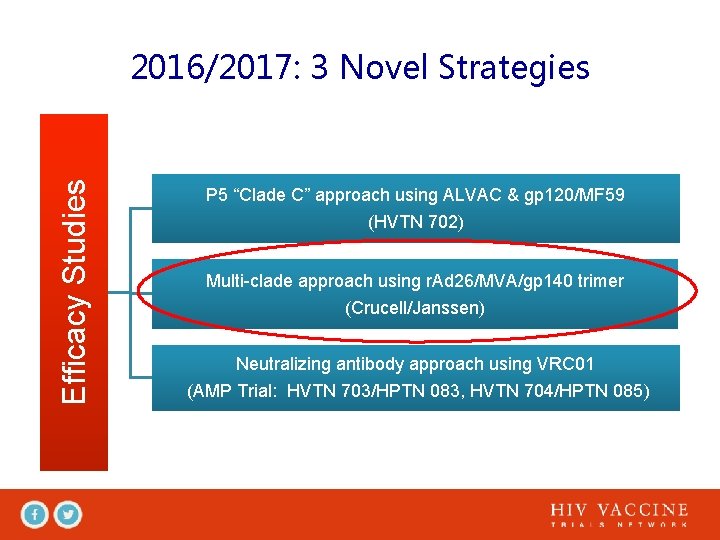
Efficacy Studies 2016/2017: 3 Novel Strategies P 5 “Clade C” approach using ALVAC & gp 120/MF 59 (HVTN 702) Multi-clade approach using r. Ad 26/MVA/gp 140 trimer (Crucell/Janssen) Neutralizing antibody approach using VRC 01 (AMP Trial: HVTN 703/HPTN 083, HVTN 704/HPTN 085)

2010 Formation of the P 5 Partnership Purpose: To build on RV 144 data and ultimately license a poxprotein based HIV vaccine with the potential for broad and timely public health impact. Strategy: Developed a partnership to extend the RV 144 concept to Clade C regions of the world. Use expert committees to select the strains and then use company expertise to manufacture these vaccines for immunogenicity, safety and efficacy.

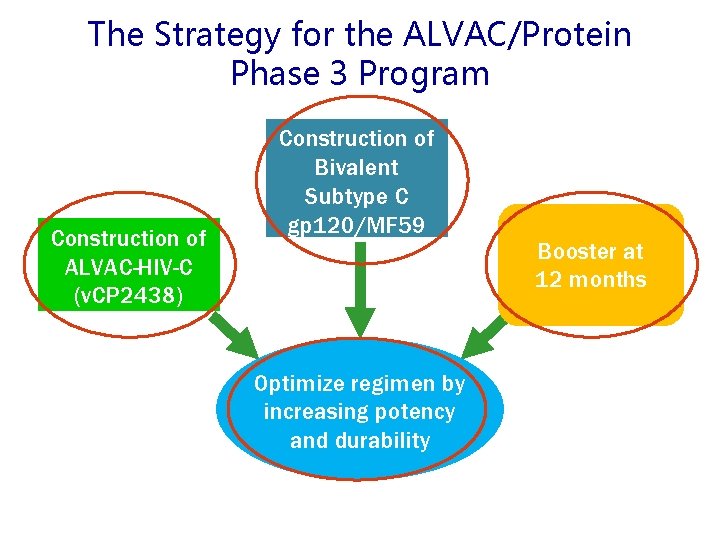
The Strategy for the ALVAC/Protein Phase 3 Program Construction of ALVAC-HIV-C (v. CP 2438) Construction of Bivalent Subtype C gp 120/MF 59 Optimize regimen by increasing potency and durability Booster at 12 months

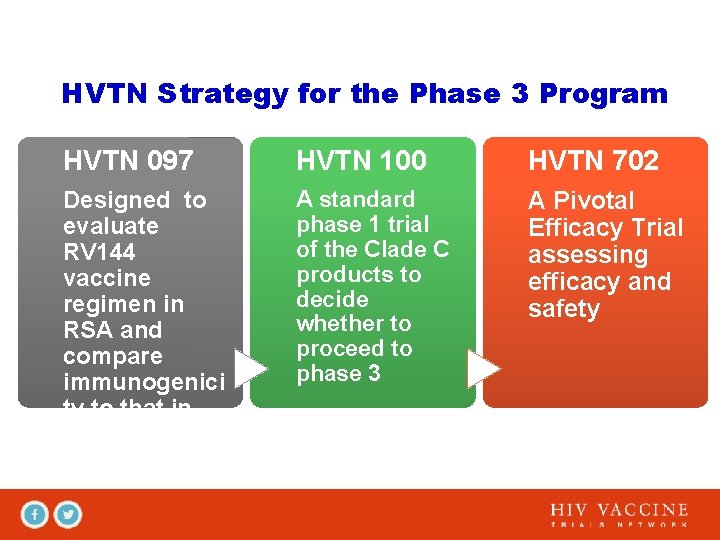
HVTN Strategy for the Phase 3 Program HVTN 097 HVTN 100 HVTN 702 Designed to evaluate RV 144 vaccine regimen in RSA and compare immunogenici ty to that in Thailand A standard phase 1 trial of the Clade C products to decide whether to proceed to phase 3 A Pivotal Efficacy Trial assessing efficacy and safety

LB xxxx Meeting the “Go” Criteria: Immunogenicity from HVTN 100, a phase 1/2 randomized, double blind, placebo-controlled trial of clade C ALVAC- ® (v. CP 2438) and Bivalent Subtype C gp 120/MF 59® in HIV-uninfected South African adults. Bekker LG, Laher F, Moodie Z, Tomaras G, Grunenberg N, Allen M, Daniels B, Innes C, Mngadi K, Malahleha M, Grant S, Gilbert P, Michael N, Phogat S, Diaz Granados C, Kanesa Thasan N, Corey L, Gray G, Mc. Elrath J, for the HVTN 100 team.

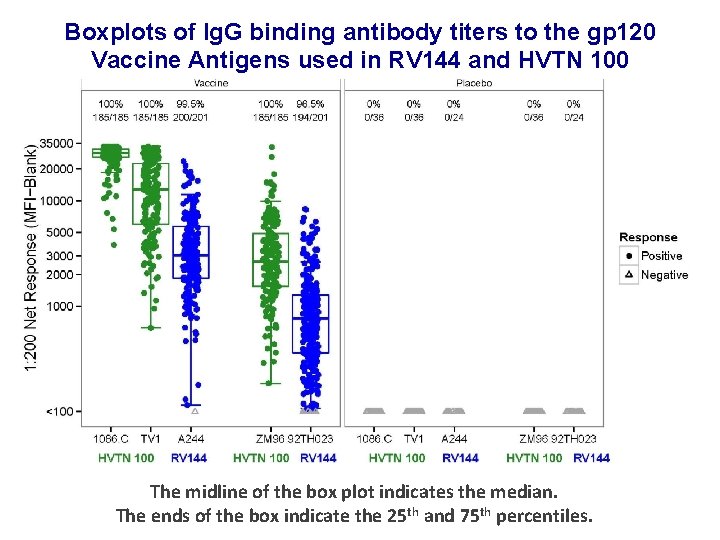
Boxplots of Ig. G binding antibody titers to the gp 120 Vaccine Antigens used in RV 144 and HVTN 100 The midline of the box plot indicates the median. The ends of the box indicate the 25 th and 75 th percentiles.

Significantly greater polyfunctionality scores in HVTN 100 (P < 0. 00001)

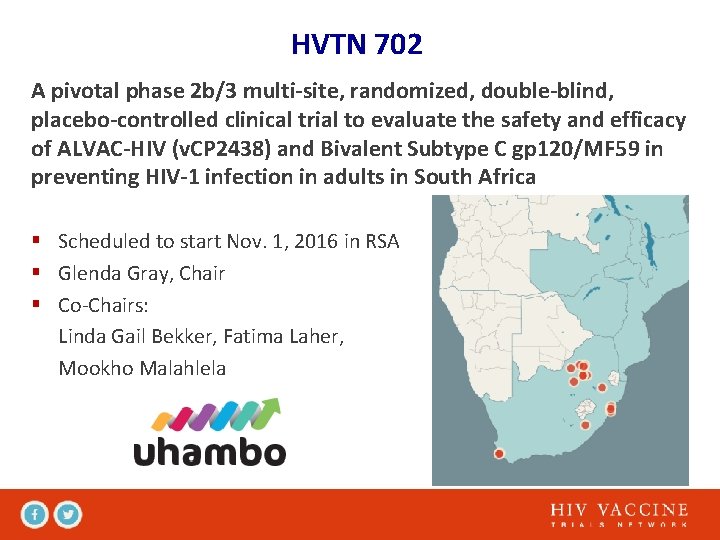
HVTN 702 A pivotal phase 2 b/3 multi-site, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of ALVAC-HIV (v. CP 2438) and Bivalent Subtype C gp 120/MF 59 in preventing HIV-1 infection in adults in South Africa § Scheduled to start Nov. 1, 2016 in RSA § Glenda Gray, Chair § Co-Chairs: Linda Gail Bekker, Fatima Laher, Mookho Malahlela

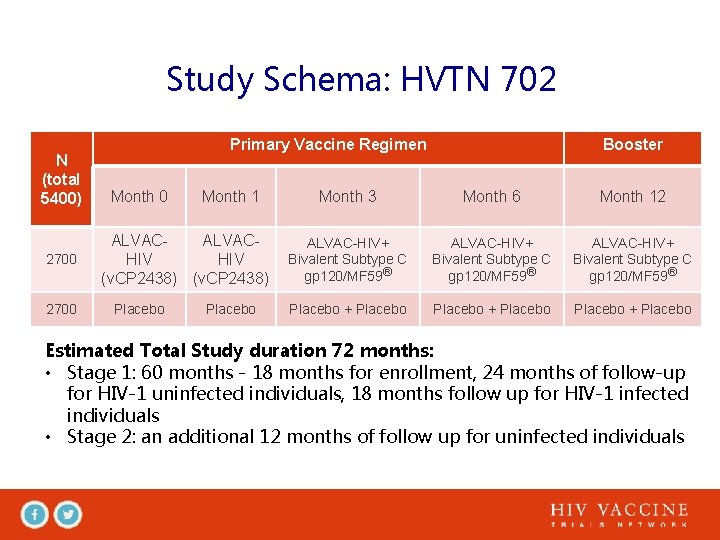
Study Schema: HVTN 702 N (total 5400) 2700 Primary Vaccine Regimen Month 0 Month 1 ALVACHIV (v. CP 2438) Placebo Booster Month 3 Month 6 Month 12 ALVAC-HIV+ Bivalent Subtype C gp 120/MF 59® Placebo + Placebo Estimated Total Study duration 72 months: • Stage 1: 60 months - 18 months for enrollment, 24 months of follow-up for HIV-1 uninfected individuals, 18 months follow up for HIV-1 infected individuals • Stage 2: an additional 12 months of follow up for uninfected individuals

Efficacy Studies 2016/2017: 3 Novel Strategies P 5 “Clade C” approach using ALVAC & gp 120/MF 59 (HVTN 702) Multi-clade approach using r. Ad 26/MVA/gp 140 trimer (Crucell/Janssen) Neutralizing antibody approach using VRC 01 (AMP Trial: HVTN 703/HPTN 083, HVTN 704/HPTN 085)

J&J HIV Vaccine Research Program: • • • BIDMC/Harvard HVTN IAVI MHRP NIAID Ragon Institute

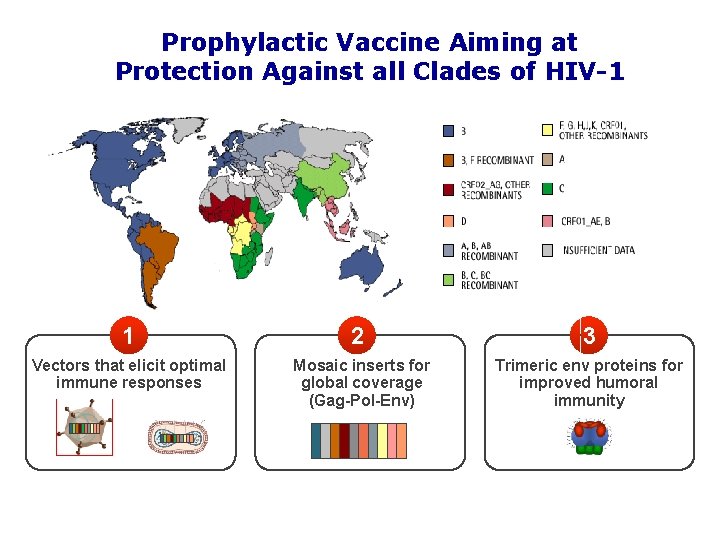
Prophylactic Vaccine Aiming at Protection Against all Clades of HIV-1 1 2 3 Vectors that elicit optimal immune responses Mosaic inserts for global coverage (Gag-Pol-Env) Trimeric env proteins for improved humoral immunity

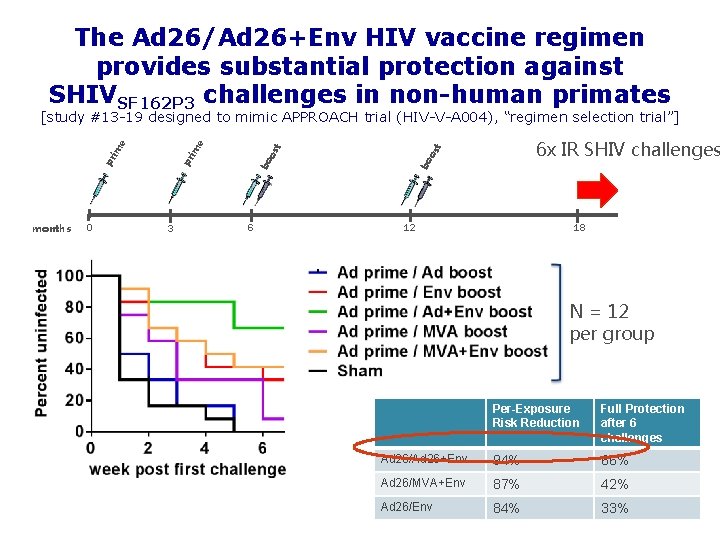
The Ad 26/Ad 26+Env HIV vaccine regimen provides substantial protection against SHIVSF 162 P 3 challenges in non-human primates months 0 3 6 t 6 x IR SHIV challenges bo os t os bo pr pr im im e e [study #13 -19 designed to mimic APPROACH trial (HIV-V-A 004), “regimen selection trial”] 12 18 N = 12 per group Per-Exposure Risk Reduction Full Protection after 6 challenges Ad 26/Ad 26+Env 94% 66% Ad 26/MVA+Env 87% 42% Ad 26/Env 84% 33%

Efficacy Studies 2016/2017: 3 Novel Strategies P 5 “Clade C” approach using ALVAC & gp 120/MF 59 (HVTN 702) Multi-clade approach using r. Ad 26/MVA/gp 140 trimer (Crucell/Janssen) Neutralizing antibody approach using VRC 01 (AMP Trial: HVTN 703/HPTN 083, HVTN 704/HPTN 085)

Long History of Antibodies to Treat and Prevent Infectious Disease (Serum Therapy) The Nobel Prize in Physiology or Medicine 1901 to Emil von Behring: “For his work on serum therapy, especially its application against diphtheria, by which he has opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and deaths". Behring together with his colleagues Wernicke (left) and Frosch (center) in Robert Koch's laboratory in Berlin. Photo: Courtesy of Aventis Behring WWW. nobelprize. org Pre-Antibiotic Era: Bering and Paul Ehrlich pioneered serum therapy for diseases such as diphtheria, tetanus, streptococcal infections Nobel Prizes awarded for discoveries related to antibodies in infectious diseases: - 1901: Serum therapy for diphtheria (Behring), - 1908: Describing humoral immunity (Mechnikov, Ehrlich), - 1972: Defining the chemical structure of antibodies (Edelman, Porter) - 1984: Production of monoclonal antibodies (m. Abs) (Jerne, Köhler, Milstein) - 1987: Explaining the mechanism for antibody diversity

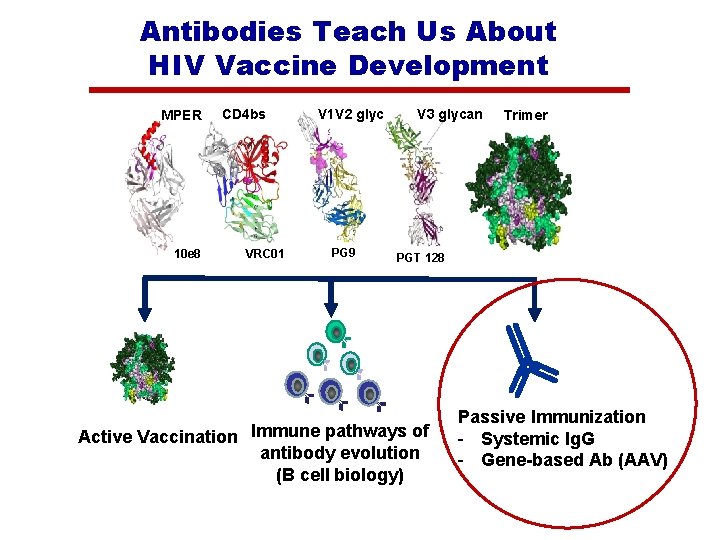
Antibodies Teach Us About HIV Vaccine Development MPER CD 4 bs V 1 V 2 glycan V 3 glycan Trimer 1 1 10 e 8 VRC 01 PG 9 PGT 128 Active Vaccination Immune pathways of antibody evolution (B cell biology) Passive Immunization - Systemic Ig. G - Gene-based Ab (AAV)

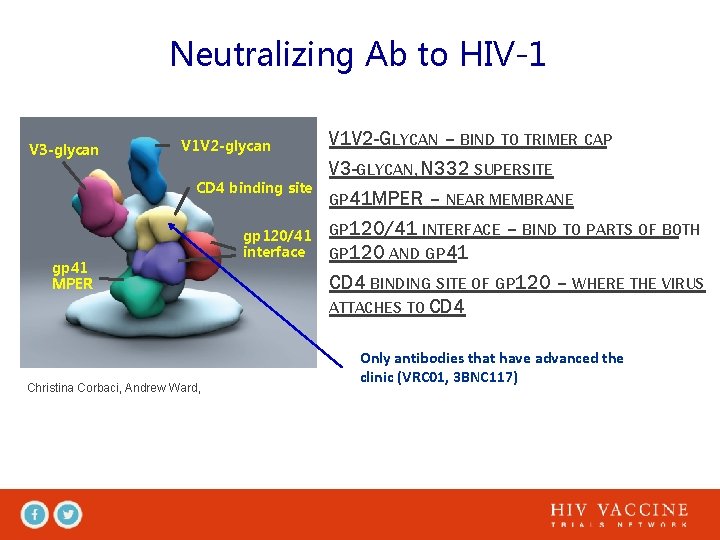
Neutralizing Ab to HIV-1 V 3 -glycan V 1 V 2 -glycan CD 4 binding site gp 41 MPER Christina Corbaci, Andrew Ward, gp 120/41 interface V 1 V 2 -GLYCAN – BIND TO TRIMER CAP V 3 -GLYCAN, N 332 SUPERSITE GP 41 MPER – NEAR MEMBRANE GP 120/41 INTERFACE – BIND TO PARTS OF BOTH GP 120 AND GP 41 CD 4 BINDING SITE OF GP 120 – WHERE THE VIRUS ATTACHES TO CD 4 Only antibodies that have advanced the clinic (VRC 01, 3 BNC 117)

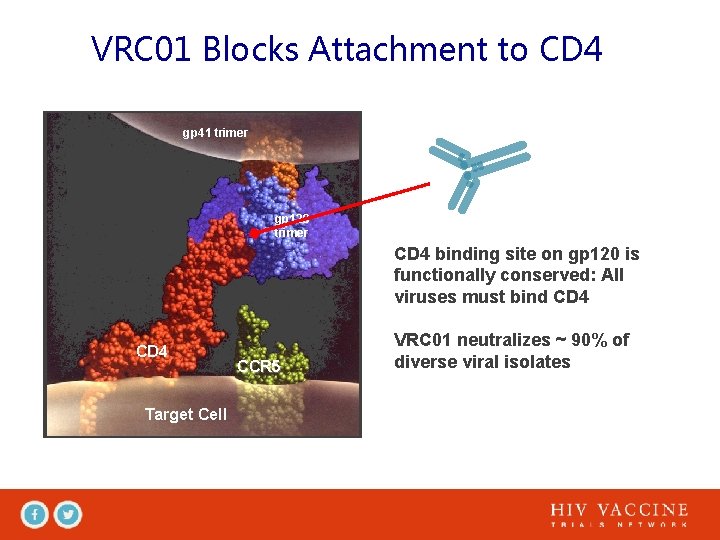
VRC 01 Blocks Attachment to CD 4 gp 41 trimer gp 120 trimer CD 4 binding site on gp 120 is functionally conserved: All viruses must bind CD 4 Target Cell CCR 5 VRC 01 neutralizes ~ 90% of diverse viral isolates

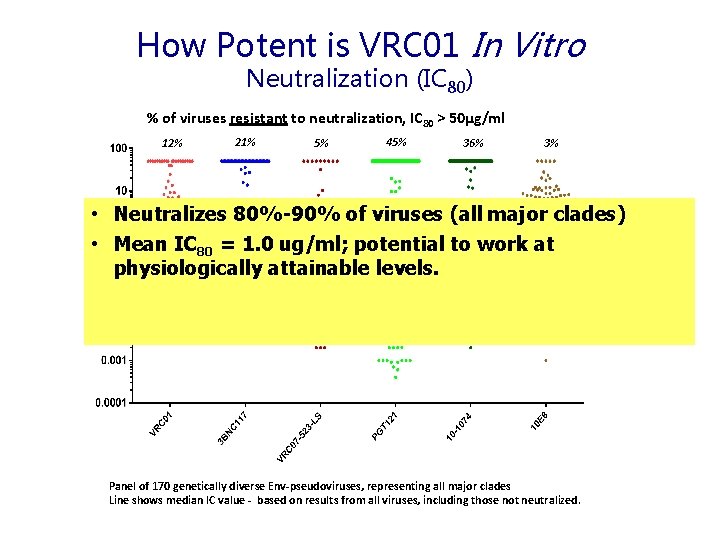
How Potent is VRC 01 In Vitro Neutralization (IC 80) % of viruses resistant to neutralization, IC 80 > 50µg/ml 12% 21% 5% 45% 36% 3% • Neutralizes 80%-90% of viruses (all major clades) • Mean IC 80 = 1. 0 ug/ml; potential to work at physiologically attainable levels. Panel of 170 genetically diverse Env-pseudoviruses, representing all major clades Line shows median IC value - based on results from all viruses, including those not neutralized.

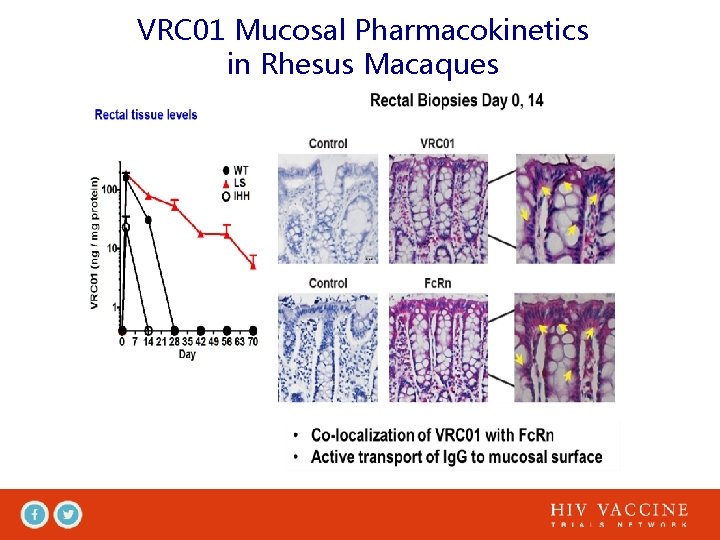
VRC 01 Mucosal Pharmacokinetics in Rhesus Macaques

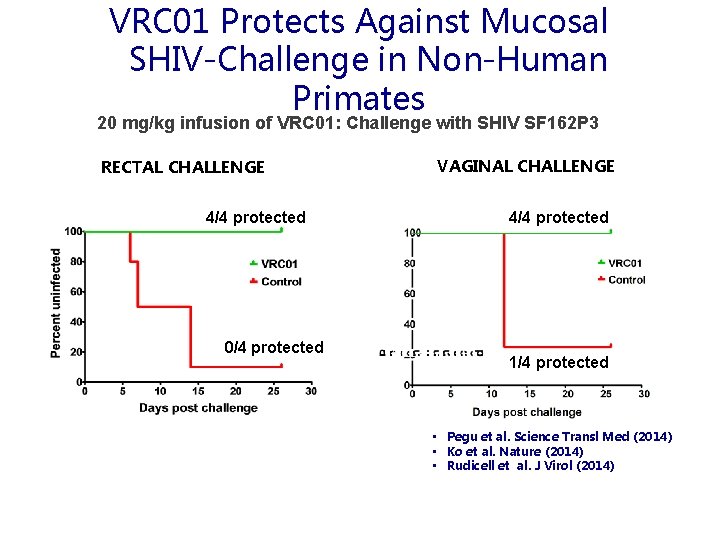
VRC 01 Protects Against Mucosal SHIV-Challenge in Non-Human Primates 20 mg/kg infusion of VRC 01: Challenge with SHIV SF 162 P 3 RECTAL CHALLENGE 4/4 protected 0/4 protected VAGINAL CHALLENGE 4/4 protected 1/4 protected • Pegu et al. Science Transl Med (2014) • Ko et al. Nature (2014) • Rudicell et al. J Virol (2014)

Passive Antibody Protection • NHP studies tell us that physiologically achievable levels of Ab could prevent HIV-1 infection • But no direct proof in humans • Learn from Proof of Concept Trial in Humans: • What level of neutralizing Ab is needed to prevent infection? o Pertains to passive b. NAb infusion or vectored delivery • Convert the m. Ab levels to serum level of neutralization needed to protect (e. g. , neut titer 1: 50, 1: 500) • Provides a benchmark for vaccine development; i. e. what antibody level does a vaccine need to achieve

Passive Antibody Prevention Phase IIB Efficacy Studies AMP = Antibody Mediated Prevention Can a passively infused monoclonal antibody prevent HIV-1 infection in high risk adults: MSM in Americas & Hetersosexual Women in sub-Saharan Africa Chairs: Lawrence Corey, HVTN Mike Cohen, HPTN Co-chairs: Srilatha Edupuganti Nyaradzo Mgodi

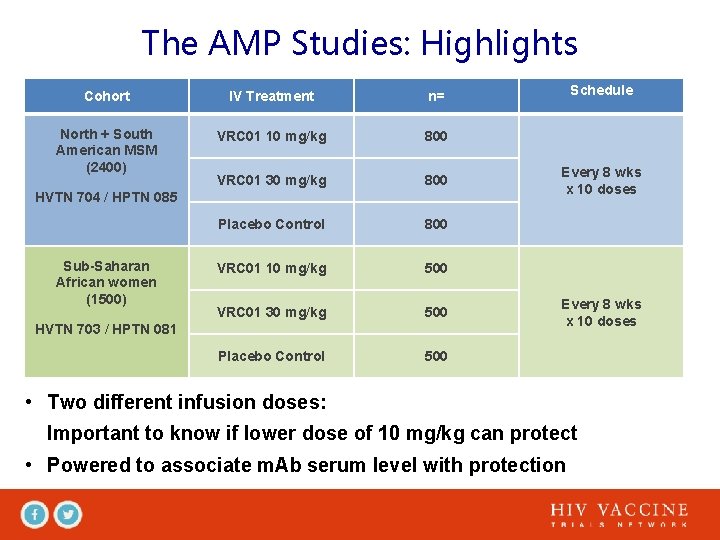
The AMP Studies: Highlights Cohort IV Treatment n= North + South American MSM (2400) VRC 01 10 mg/kg 800 VRC 01 30 mg/kg 800 Placebo Control 800 VRC 01 10 mg/kg 500 VRC 01 30 mg/kg 500 Placebo Control 500 HVTN 704 / HPTN 085 Sub-Saharan African women (1500) HVTN 703 / HPTN 081 Schedule Every 8 wks x 10 doses • Two different infusion doses: Important to know if lower dose of 10 mg/kg can protect • Powered to associate m. Ab serum level with protection

Rationale for the 2 Cohorts and 2 Dosing Regimens • Route of acquisition and genital tract immunology and anatomy may influence the distribution of VRC 01 and potential efficacy. • Variation in the doses allows us to more precisely define what are the optimal concentrations of neutralizing activity associated with protection from acquisition.

66% Overlap in Concentrations 62. 5% PYRs

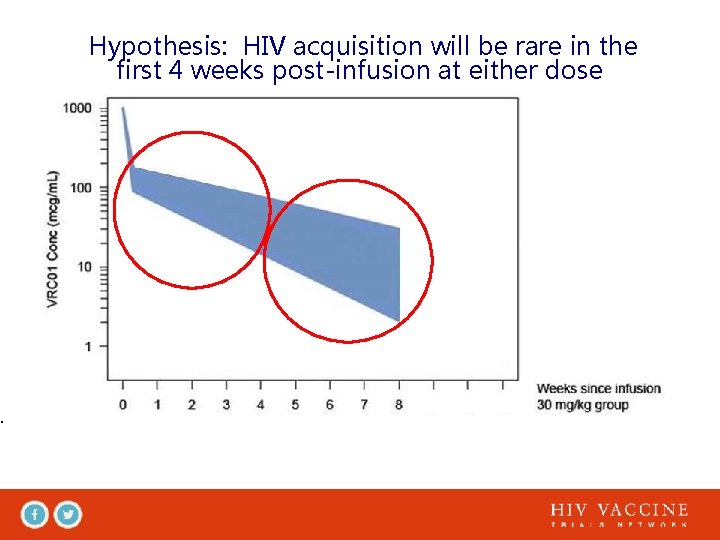
Hypothesis: HIV acquisition will be rare in the first 4 weeks post-infusion at either dose

Concepts from the AMP Studies �Can lower levels of neutralization activity afford protection or does in vivo protection require only high concentrations of CD 4 binding site antibodies? �Are non-neutralizing effector functions as predictive of efficacy as neutralizing activity? • Defining the optimal concentration defining efficacy allows one to engineer a second generation product and delivery system that will provide protective levels in a way that can be delivered efficiently on a population basis. • Higher potency; longer half-life; cheaper delivery.

Potential Role in Interruption of Maternal to Child Transmission Could HIV m. Abs: q Protect against infection resulting from intrapartum exposure to HIV; i. e. , during childbirth q Protect the infant during the course of breastfeeding (months)

A Pictorial History of HIV-1 Vaccine Efficacy Trials 1995 2000 2005 1 year Vax. Gen IDU Thai Trial (gp 120) 2015 2020 702 IAS Durban Vax. Gen USA (gp 120) 2010 Step Trial/Phambili Trials (Ad 5 gag/pol/nef) RV 144 Thai Trial (ALVAC/gp 120) HVTN 505 (DNA/Ad 5 env/gag/pol AMP Trial (VRC-01) HVTN 702 (Clade C ALVAC/gp 120) Janssen Test of Concept Trial start/end Trial analysis/results Immune correlates First data available AMP Janssen Trial

61

62

The HIV Vaccine Field is Open for Business • Three pivotal HIV vaccine related efficacy trials are either in progress (AMP) or soon to be initiated: HVTN 702 and Janssen POC trial in 2016 - 2017. • These pivotal efficacy studies will define if either or both neutralizing and/or non-neutralizing antibodies can be tweaked to provide reasonable vaccine efficacy in high risk Clade C regions of the world. • These studies will set the stage for the entire design and development of HIV vaccines for the next decade.

The HIV Vaccine Field is in a Novel Space • Using human clinical trials with intense evaluation of the Correlates of Protection is in the end - true “rational vaccine design”. • For the first time, the basic science agenda in HIV vaccine development will be based on human clinical trials.

Acknowledgments HVTN Lab Program Julie Mc. Elrath, Georgia Tomaras, Nicole Frahm, John Hural, David Montefiori, Steve De. Rosa, Erica Andersen-Nissen, Lynn Morris USMHRP Nelson Michael, Robert O’Connell Bill and Melinda Gates Foundation Emilio Emini, Nina Russell and Sanofi Pasteur team Jim Tartaglia, Sanjay Gurunathan, Sanjay Phogat Janssen Frank Tomaka, Maria Pau, Hanneke Schuitemaker, Paul Stoffels HVTN Core, SDMC, EMT Jim Kublin, Peter Gilbert, Glenda Gray, Susan Buchbinder, Scott Hammer, Gepi Pantaleo, Shelly Karuna, Nicole Grunenberg, Carter Bentley Site Investigators Study Volunteers CHAVI ID Bart Haynes, Larry Liao and colleagues DAIDS Vaccine Research Program Carl Dieffenbach, Mary Marovich, Dale Hu, Phil Renzullo, Pat D’Souza, Paul Kitsutani, Mary Allen, Jim Lane, Mike Pensiero

Collaborators - Africa • • • Glenda Gray Linda Gail-Bekker Gita Ramjee Cheryl Louw Kathy Mngadi Graeme Meintjes Craig Innes Nicole Hunt Phillip Kotze Francis Martinson • • • Jani Ilesh Stewart Reid Leonard Maboko Maphoshane Nchabeleng Lungiswa Mtingi Dumezweni Ntshangase William Brumskine Zvavahera Chirenje Mookho Malahlela Modulakgotla Sebe

Collaborators - U. S. , South America and Europe • • • Mark Mulligan Paul Goepfert Ray Dolin Lindsey Baden Ken Mayer Richard Novak Benigno Rodriguez Spyros Kalams Scott Hammer • • • Beryl Koblin Ian Frank Michael Keefer Susan Buchbinder Julie Mc. Elrath Gepi Pantaleo Jorge Sanchez Martin Casapia Robinson Cabello
 Physical progress and financial progress
Physical progress and financial progress Gavin corey
Gavin corey Betty parris the crucible character traits
Betty parris the crucible character traits Hkn uiuc
Hkn uiuc Corey snyder uiuc
Corey snyder uiuc Corey fehr
Corey fehr The crucible act 1 characters
The crucible act 1 characters Nominative address
Nominative address Mary warren quote
Mary warren quote Ece 313
Ece 313 Dr corey beck
Dr corey beck Cwu clubs
Cwu clubs Marshal herrick personality traits
Marshal herrick personality traits Teen learning lab
Teen learning lab What is the conflict in the crucible act 2
What is the conflict in the crucible act 2 Corey kendig
Corey kendig Corey bergeron
Corey bergeron Ej corey
Ej corey Corey
Corey The crucible final exam
The crucible final exam Crucible def
Crucible def Individual differences factors
Individual differences factors Database field types and field properties
Database field types and field properties Field dependent vs field independent
Field dependent vs field independent Field dependent and field independent
Field dependent and field independent Electric field and magnetic field difference
Electric field and magnetic field difference Difference between electric field and magnetic field
Difference between electric field and magnetic field 21lwuy8i6hw -site:youtube.com
21lwuy8i6hw -site:youtube.com E field h field
E field h field Klamydia syntom
Klamydia syntom Triệu chứng nhiễm hiv
Triệu chứng nhiễm hiv Hiv transmissions
Hiv transmissions Hiv index testing steps
Hiv index testing steps Dot resposta
Dot resposta Hiv in adults
Hiv in adults Alur pelayanan hiv di puskesmas
Alur pelayanan hiv di puskesmas Hiv test window period
Hiv test window period Hiv
Hiv Ephi ethiopia
Ephi ethiopia Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Ciclo do hiv
Ciclo do hiv Hiv vaccinr
Hiv vaccinr Window period hiv
Window period hiv Hiv
Hiv Patogenesis hiv
Patogenesis hiv Hiv patologia
Hiv patologia Hiv lifecycle
Hiv lifecycle Hiv
Hiv Most common malignancy in hiv
Most common malignancy in hiv Risk of blood transfusion
Risk of blood transfusion Causative organism of hiv/aids
Causative organism of hiv/aids Objawy hiv
Objawy hiv Hiv retrovirus
Hiv retrovirus Nsradio
Nsradio Global hiv prevention coalition
Global hiv prevention coalition Hiv risk factors
Hiv risk factors Hiv family name
Hiv family name Hiv
Hiv Standar program nasional 5
Standar program nasional 5 Vidas hiv duo ultra package insert
Vidas hiv duo ultra package insert Hiv virus
Hiv virus Yu1u
Yu1u