Programmed Learning Material Developed By Dr FAIZA ALTAF

Programmed Learning Material Developed By Dr. FAIZA ALTAF Research Scholar(JMI) Click here to start

Description of Programmed Learning Material • Programmed Learning is a technique of auto instruction. It gives you an opportunity to learn at their own by providing better communication between the instructional material and the learner. • This is a computer based Programmed Learning Material. • This Programmed Learning Material is based on chapter namely “Matter in our surroundings ” of class IX science text book prescribed by NCERT. • There are total 21 Frames in this PLM.

Behavioral Objectives---After studying with this PLM students will be able to------ • Recognize the different types of Matter. • Describe the physical properties of Matter. • Explain the characteristics of particles of Matter. • Differentiate between states of Matter. • Analyze the inter conversion of states of Matter into each other. • Illustrate the process of Evaporation in Liquids. • Define Plasma State of Matter and Bose-Einstein State of Matter.
Instructions for the learner • First you have to read each frame carefully and choose the correct answers of the questions from options given in the boxes. • After giving the answers you will get the responses like “congratulations” or “try again”. • If your answer will be correct then go to the next frame by clicking on the sign given on the right side of each frame. • If your answer will be wrong then read the frame again. • Symbols Used ---- Home Previous Next Reload
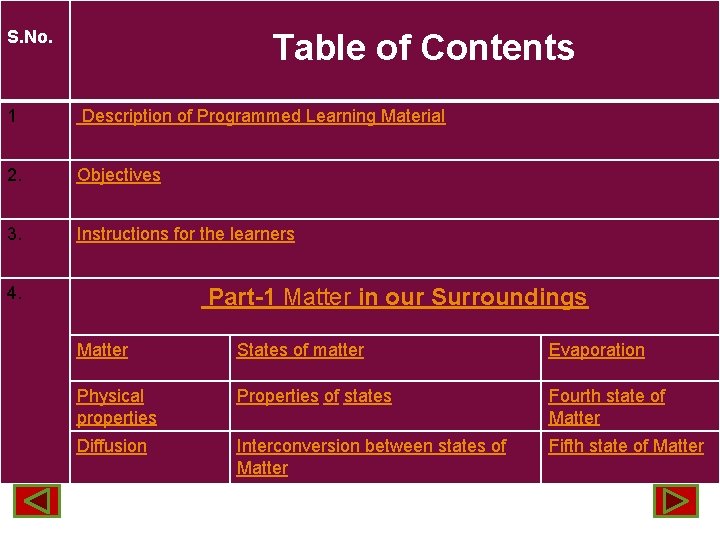
S. No. Table of Contents 1 Description of Programmed Learning Material 2. Objectives 3. Instructions for the learners 4. Part-1 Matter in our Surroundings Matter States of matter Evaporation Physical properties Properties of states Fourth state of Matter Diffusion Interconversion between states of Matter Fifth state of Matter

Matter In our Surroundings Click here to Start
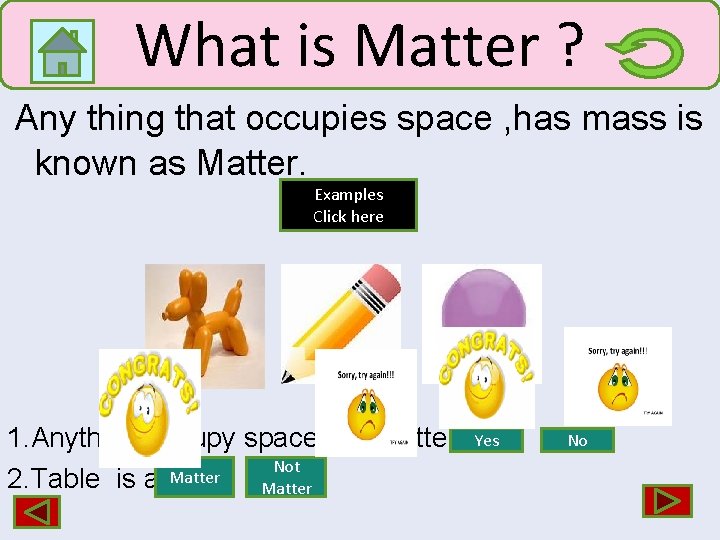
What is Matter ? Any thing that occupies space , has mass is known as Matter. Examples Click here 1. Anything occupy space is a matter. Not 2. Table is a Matter Yes No
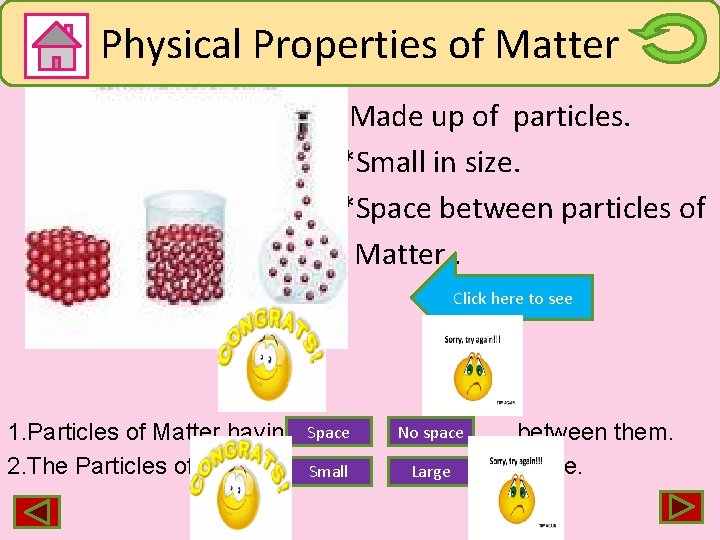
Physical properties of Matter Physical Properties of Matter *Made up of particles. *Small in size. *Space between particles of Matter. Click here to see 1. Particles of Matter having Space 2. The Particles of Matter are. Small No space Large between them. in size.
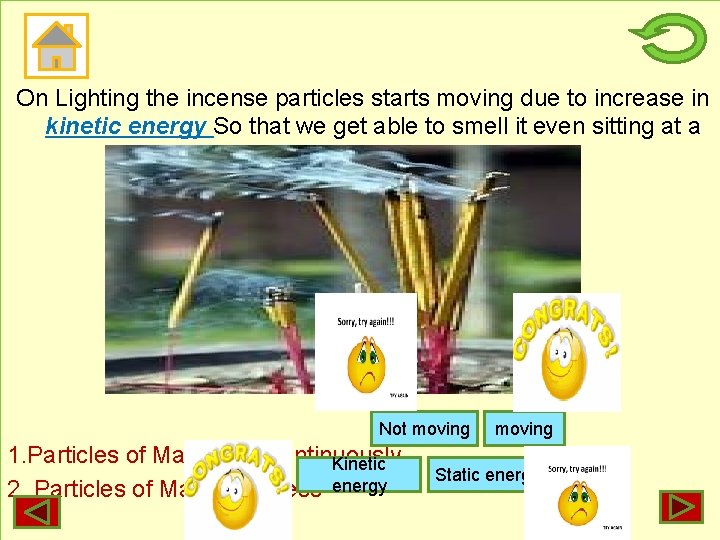
On Lighting the incense particles starts moving due to increase in kinetic energy So that we get able to smell it even sitting at a distance. Not moving 1. Particles of Matter are continuously Kinetic 2. Particles of Matter possess energy moving Static energy
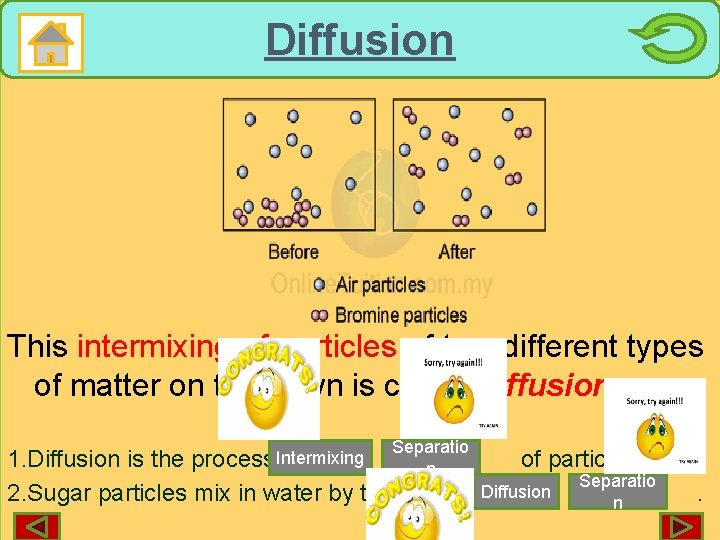
Diffusion This intermixing of particles of two different types of matter on their own is called diffusion. Separatio 1. Diffusion is the process Intermixing of of particles. n Separatio Diffusion 2. Sugar particles mix in water by the process of n .
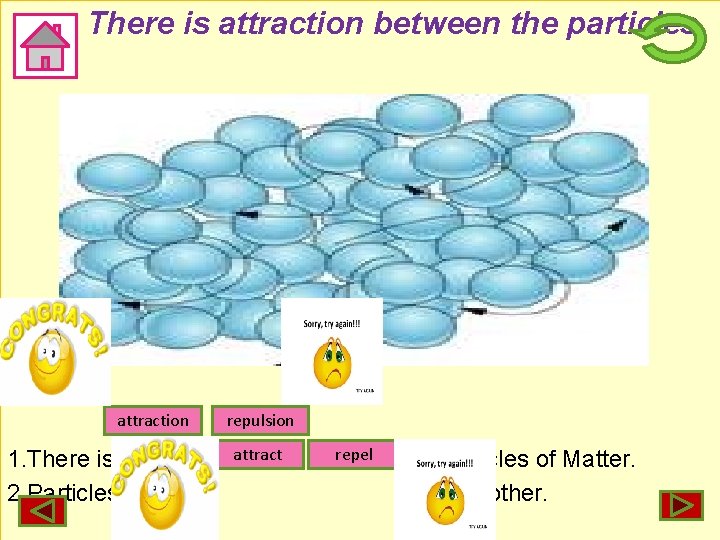
There is attraction between the particles of Matter. attraction 1. There is 2. Particles of Water repulsion attract repel between Particles of Matter. each other.
States of Matter 6 1. There are 3 states of matter. 2. Ice, Water and Water Vapour are three states of Water Air
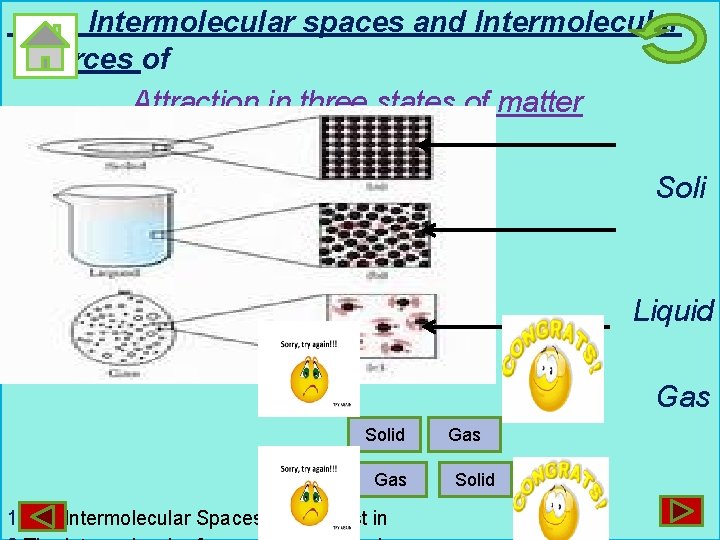
Intermolecular spaces and Intermolecular Forces of Attraction in three states of matter Soli d Liquid Gas Solid Gas 1. The Intermolecular Spaces are highest in Gas Solid
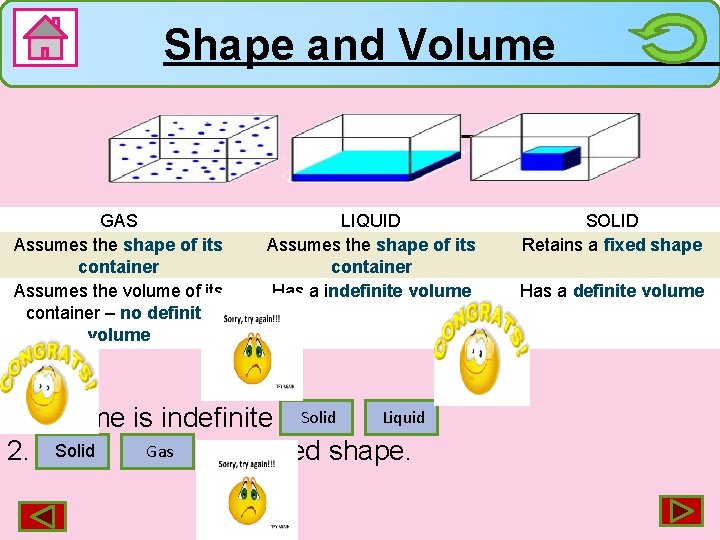
Shape and Volume GAS Assumes the shape of its container Assumes the volume of its container – no definite volume LIQUID Assumes the shape of its container Has a indefinite volume Liquid 1. Volume is indefinite in. Solid Gas 2. Solid has fixed shape. SOLID Retains a fixed shape Has a definite volume
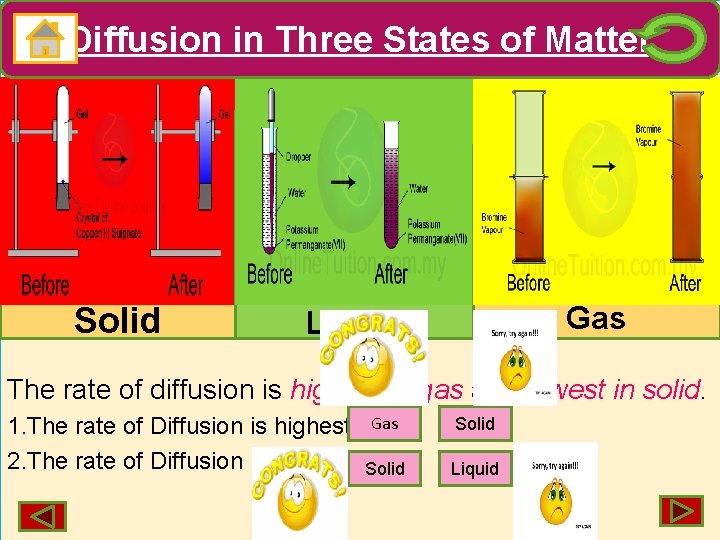
Diffusion in Three States of Matter Solid Gas Liquid The rate of diffusion is highest in gas and lowest in solid. 1. The rate of Diffusion is highest in. Gas 2. The rate of Diffusion is lowest in. Solid Liquid
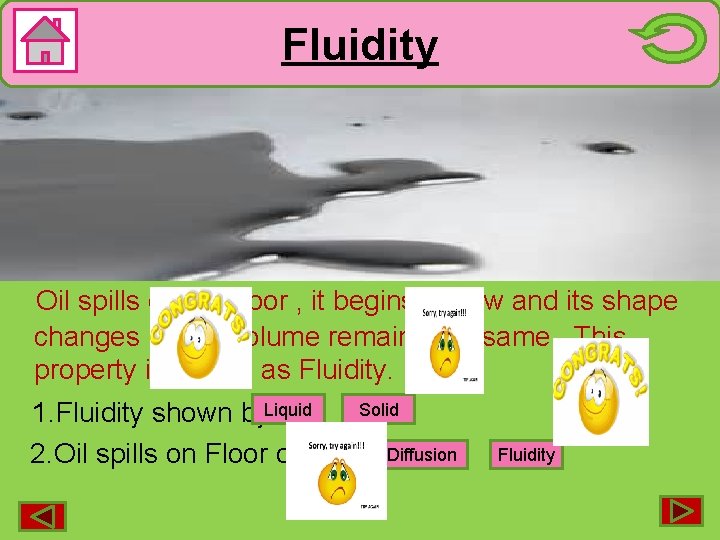
Fluidity (shown by Liquids) Oil spills on the floor , it begins to flow and its shape changes but its volume remains the same. This property is known as Fluidity. Solid 1. Fluidity shown by. Liquid 2. Oil spills on Floor due to Diffusion Fluidity
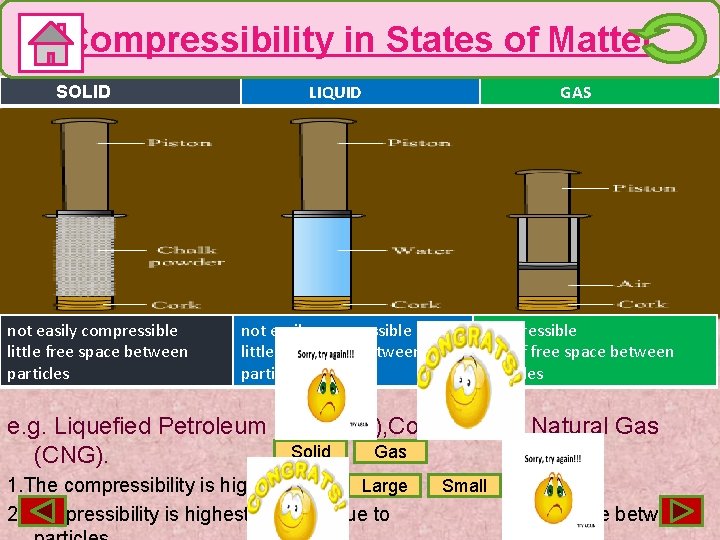
Compressibility in States of Matter SOLID not easily compressible little free space between particles LIQUID GAS not easily compressible little free space between particles compressible lots of free space between particles e. g. Liquefied Petroleum Gas(LPG), Compressed Natural Gas Solid Gas (CNG). Large 1. The compressibility is highest in 2. Compressibility is highest in gases due to Small space between
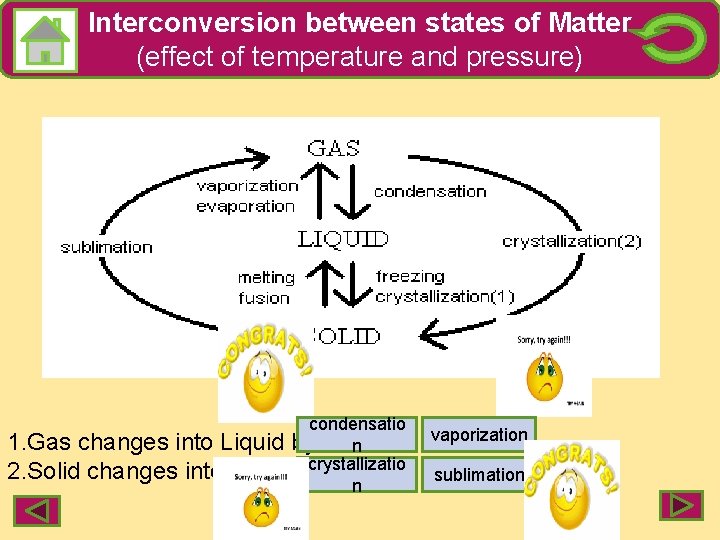
Interconversion between states of Matter (effect of temperature and pressure) condensatio 1. Gas changes into Liquid by n 2. Solid changes into Gas by crystallizatio n vaporization sublimation
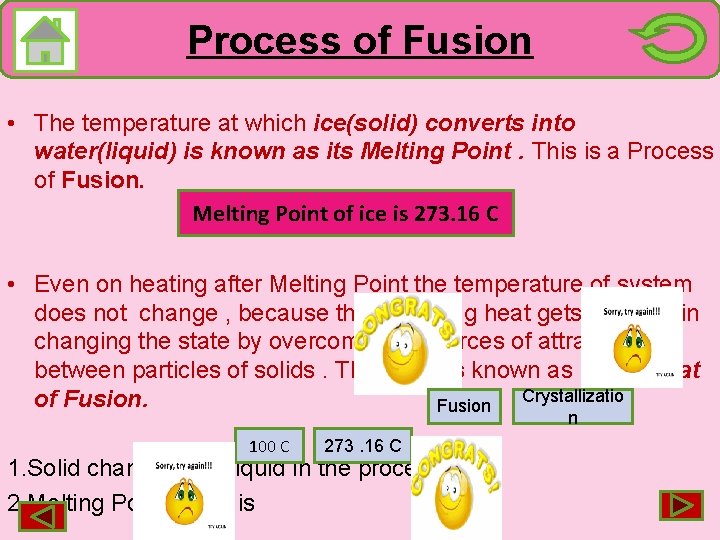
Process of Fusion • The temperature at which ice(solid) converts into water(liquid) is known as its Melting Point. This is a Process of Fusion. Melting Point of ice is 273. 16 C • Even on heating after Melting Point the temperature of system does not change , because the supplying heat gets used up in changing the state by overcoming the forces of attraction between particles of solids. This heat is known as latent heat Crystallizatio of Fusion n 100 C 273. 16 C 1. Solid changes into liquid in the process of 2. Melting Point of Ice is
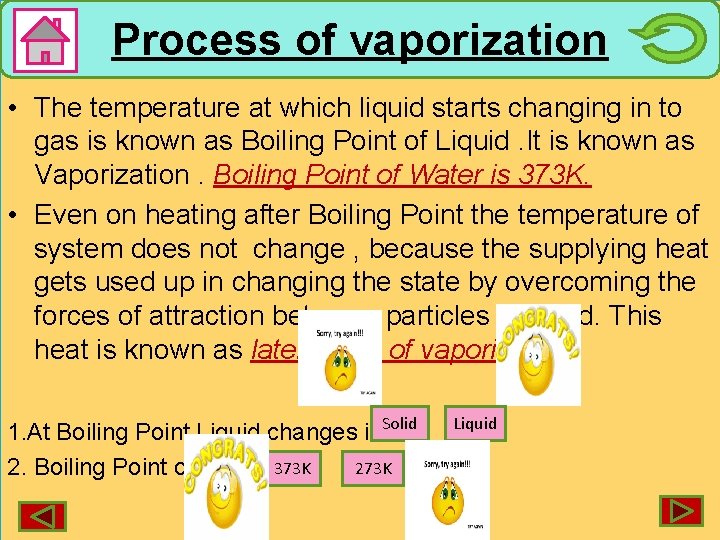
Process of vaporization • The temperature at which liquid starts changing in to gas is known as Boiling Point of Liquid. It is known as Vaporization. Boiling Point of Water is 373 K. • Even on heating after Boiling Point the temperature of system does not change , because the supplying heat gets used up in changing the state by overcoming the forces of attraction between particles of liquid. This heat is known as latent heat of vaporization. Solid 1. At Boiling Point Liquid changes into 273 K 2. Boiling Point of water is 373 K Liquid
How to convert Temperature Scales? We can convert temperature in to Celsius and Kelvin scale as— • 300 Kelvin convert into Celsius scale by------300 K- 273=27 C • 150 C convert into Kelvin scale by-----153 C+273=426 K 300 C 1. Convert 573 K into Celsius scale 2. Convert 250 C into Kelvin Scale 523 K 846 C 23 K
Evaporatio n Click here Evaporation Change of a liquid into vapour at any temperature below its boiling point is called Evaporation causes 1. In Evaporation water converts into. Vapour Evaporatio Flying 2. Clothes dry in Air due to n Cooling. Ice
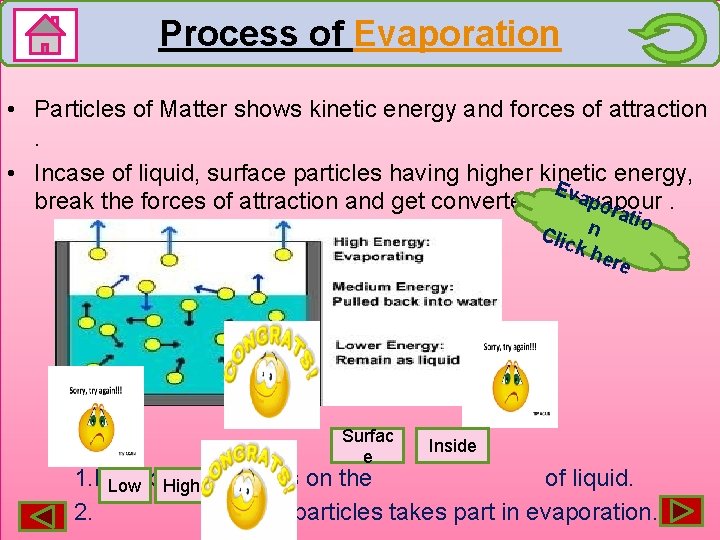
Process of Evaporation • Particles of Matter shows kinetic energy and forces of attraction. • Incase of liquid, surface particles having higher kinetic energy, Eva break the forces of attraction and get converted into pvapour. ora tio n Clic kh ere Surfac e Inside 1. Evaporation occurs on the of liquid. Low High 2. energy particles takes part in evaporation.
Factors affecting the Evaporation • Surface area-- increases Evaporation -increases • Temperature -- increases Evaporation -increases • Humidity -- increases Evaporation -decreases Decrease • Wind Speed – increases. Increase. Evaporation -s s increases Decreas es Increases 1. In Summers temperature increases then rate of evaporation -
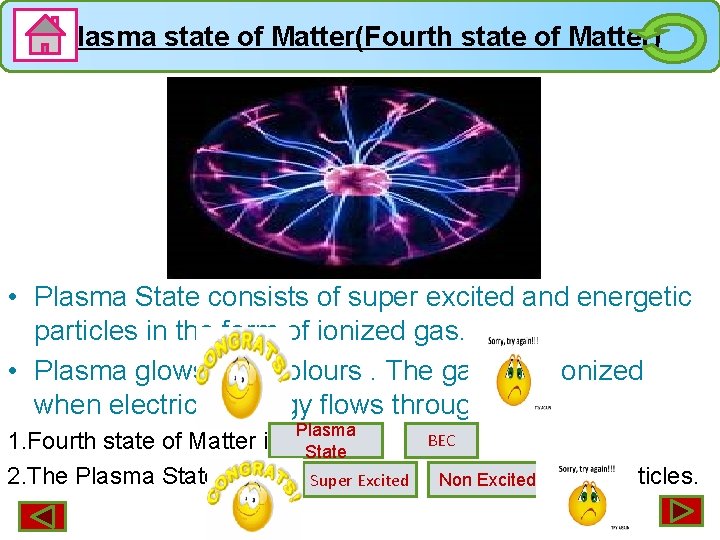
Plasma state of Matter(Fourth state of Matter) • Plasma State consists of super excited and energetic particles in the form of ionized gas. • Plasma glows with colours. The gas gets ionized when electrical energy flows through it. Plasma 1. Fourth state of Matter is State 2. The Plasma State consists. Super of Excited BEC Non Excited particles.
Fifth state of Matter. • In 1920 Indian Physicist Satyendra Nath Bose did some calculations for fifth state of Matter. • Albert Einstein predicted a new state of Matter-the Bose Einstein Condensate (BEC). • In 2001, Eric A. Cornell, Wolfgang Ketterel and Carl E. Wieman of USA received the Nobel prize in Physics for BEC. Satyendra Nath Eric A. Cornell 1. Calculation of Fifth state done by Bose Satyendra Nath Einstein 2. Bose Einstein Condensate Predicted by Bose
- Slides: 26