Prof Kokil Dhananjay Narayan Dept of Chemistry Class









- Slides: 22


Prof. Kokil Dhananjay Narayan Dept. of Chemistry Class : S. Y. B. Sc. Paper III {Organic Chemistry}

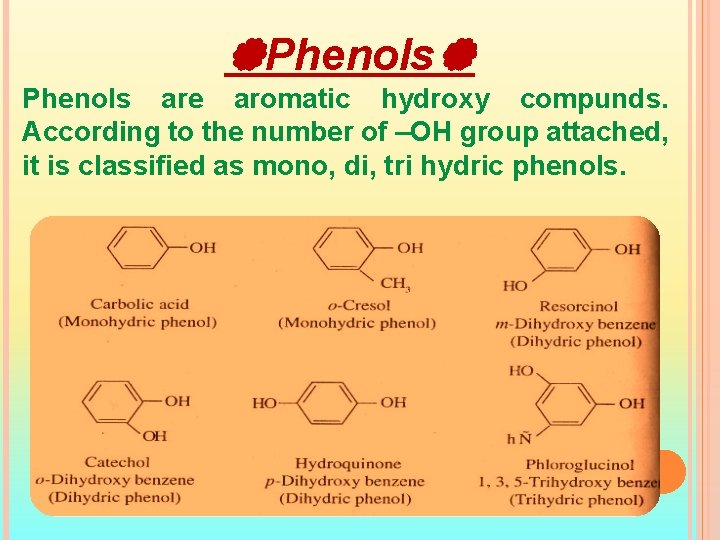
Phenols are aromatic hydroxy compunds. According to the number of –OH group attached, it is classified as mono, di, tri hydric phenols.

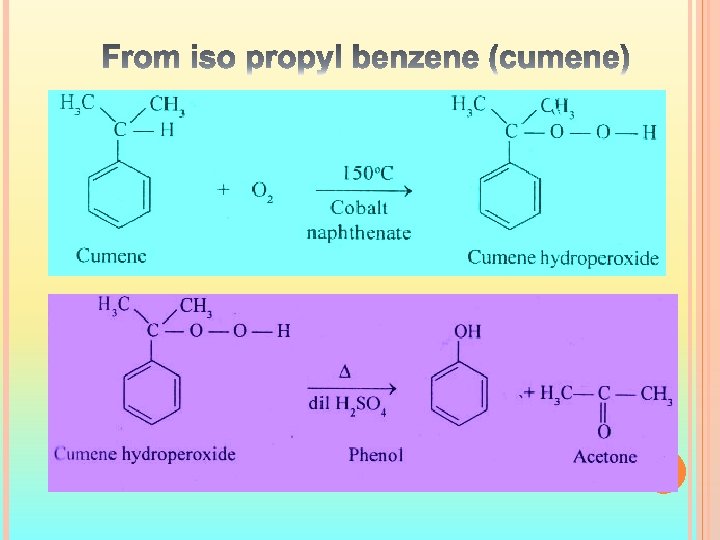
Preparation of Phenol : There are three methods 1) From Haloarenes 2) From aromatic sulphonic acids 3) From isopropyl benzene 1) From Haloarenes : - SN 1 and SN² reactions are not possible for haloarenes because of electron withdrawing nature of phenyl ring gives the positive charge to halogen.

But under drastic condition of temp 300ºc & pressure it reacts with Na. OH to give phenol.

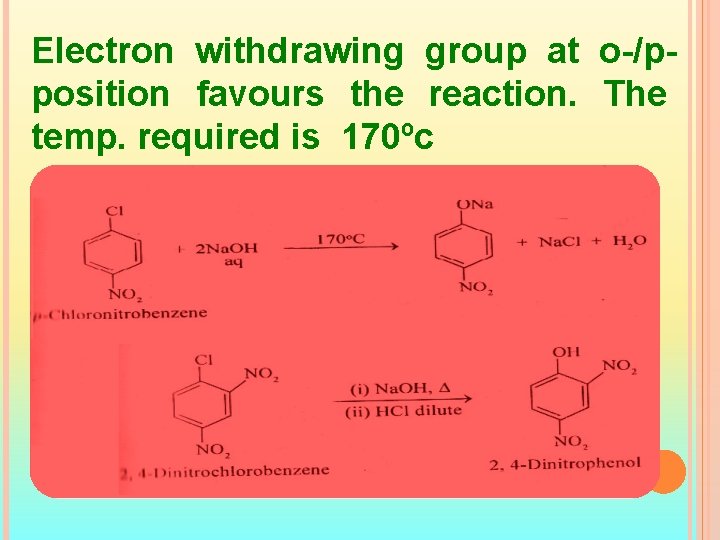
Electron withdrawing group at o-/pposition favours the reaction. The temp. required is 170ºc

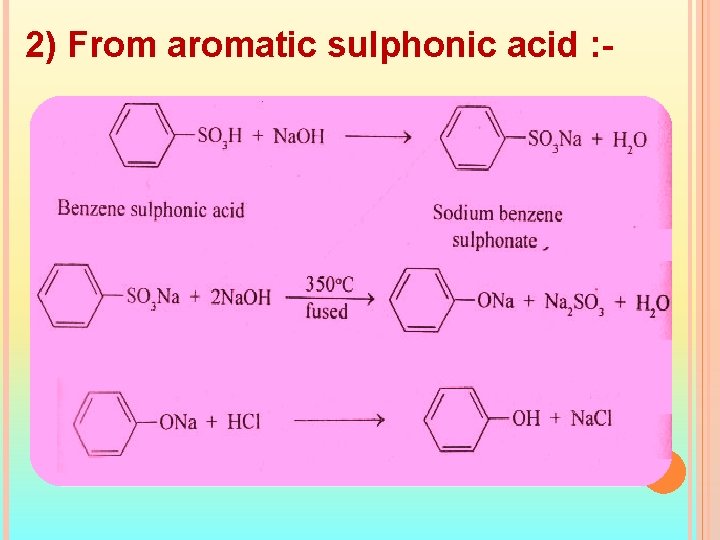
2) From aromatic sulphonic acid : -


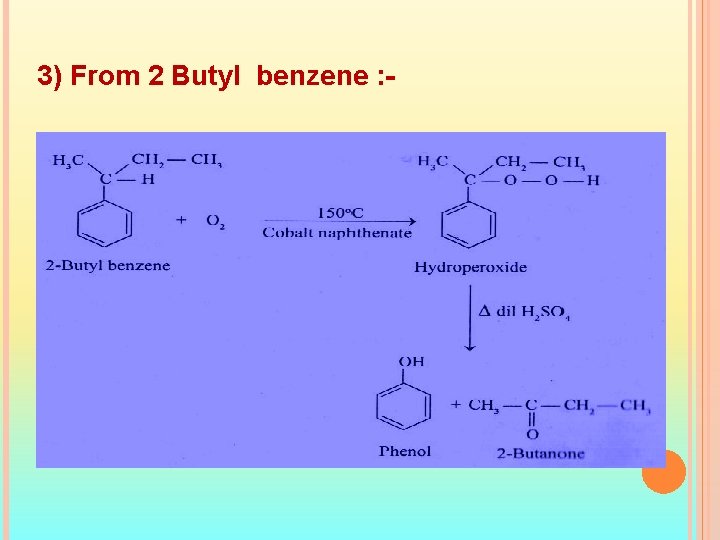
3) From 2 Butyl benzene : -

Acidity of Phenol : Electron withdrawing group like -NO 2, Cl, -Br, -I, increased the acidity of phenol while Electron donating group like –CH 3 (alkyl groups), -OCH 3 (alkoxy groups) decreases the acidity phenol. i. e. Electron withdrawing group weakens the bond between O-H of phenol while Electron donating group strengthen the bond between O-H of phenol.


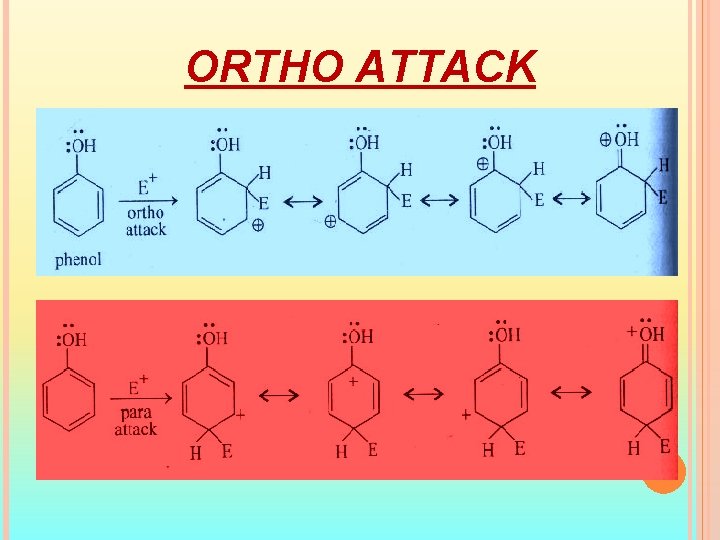
ORTHO ATTACK

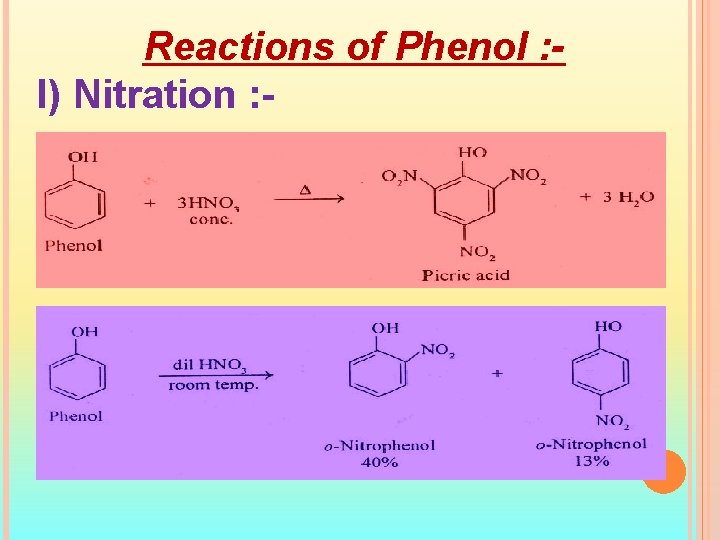
Reactions of Phenol : I) Nitration : -

II) Halogenation : -

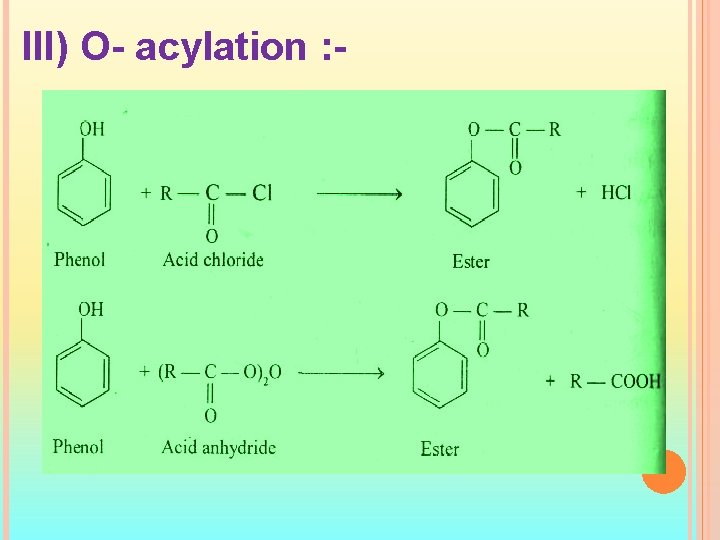
III) O- acylation : -

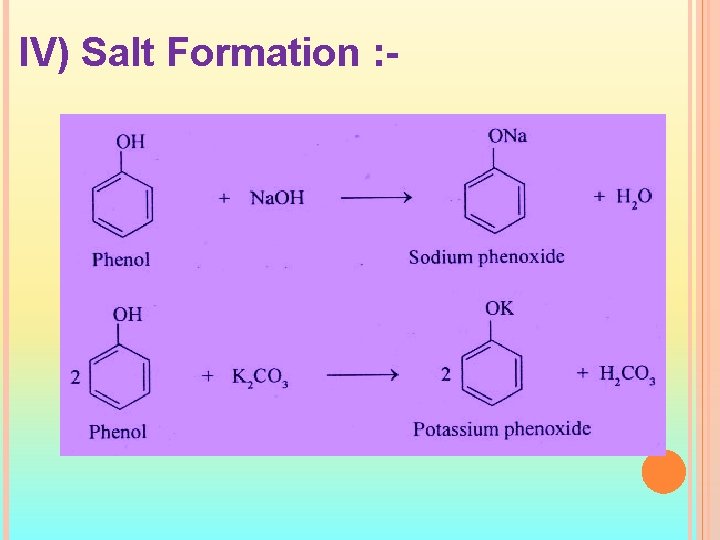
IV) Salt Formation : -

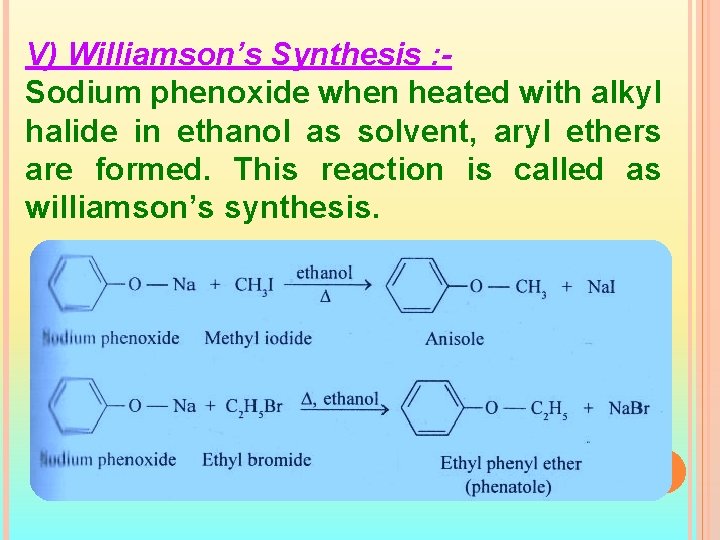
V) Williamson’s Synthesis : Sodium phenoxide when heated with alkyl halide in ethanol as solvent, aryl ethers are formed. This reaction is called as williamson’s synthesis.

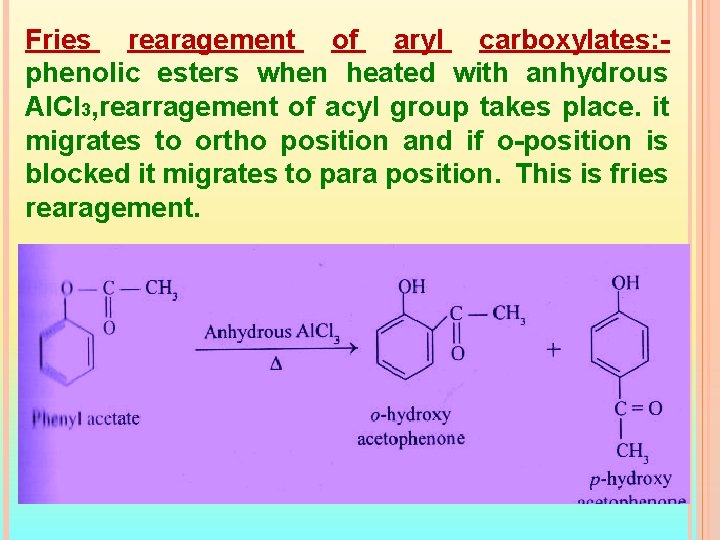
Fries rearagement of aryl carboxylates: phenolic esters when heated with anhydrous Al. Cl 3, rearragement of acyl group takes place. it migrates to ortho position and if o-position is blocked it migrates to para position. This is fries rearagement.

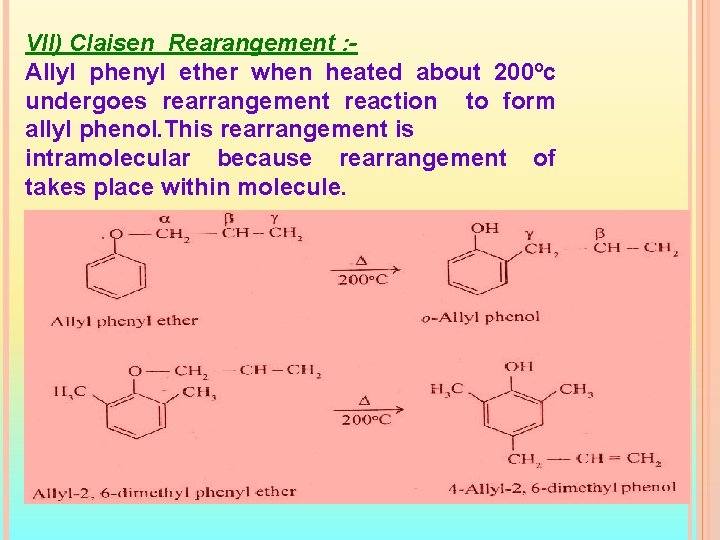
VII) Claisen Rearangement : Allyl phenyl ether when heated about 200ºc undergoes rearrangement reaction to form allyl phenol. This rearrangement is intramolecular because rearrangement of takes place within molecule.

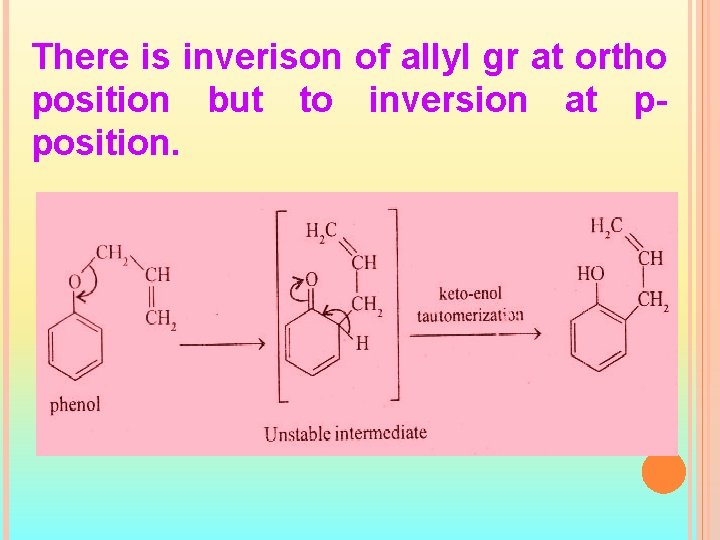
There is inverison of allyl gr at ortho position but to inversion at pposition.

Applications of Phenols : 1) It is used for the manufacture of salicylic acid, aspirin, methyl salicylate. 2) It is used to prepare dyes e. g. alizarin, orange II etc. 3) It is used to prepare phenolic resins used in adhesives & plastics. 4) It is used to prepare powerful antiseptic dettol [2, 4 dichloro, 3, 5 dimethyl phenol 5) It is used for preparation of cyclo-hexanol which is used in the production of nylon.

 Sndp movement full form
Sndp movement full form Narayan motors
Narayan motors Summary of hundred dresses 1
Summary of hundred dresses 1 Narayan mandayam
Narayan mandayam Brock
Brock Purdah poem
Purdah poem Dept nmr spectroscopy
Dept nmr spectroscopy Florida department of agriculture and consumer services
Florida department of agriculture and consumer services Finance department organizational chart
Finance department organizational chart Worcester building dept
Worcester building dept Dept. name of organization
Dept. name of organization Mn dept of education
Mn dept of education Ms department of finance and administration
Ms department of finance and administration Dept. name of organization
Dept. name of organization Ohio employment first
Ohio employment first Hjdkdkd
Hjdkdkd Vaginal dept
Vaginal dept Gome dept
Gome dept Gome dept
Gome dept Nyttofunktion
Nyttofunktion Gome dept
Gome dept Hoe dept
Hoe dept La city fire interview
La city fire interview