prof Ivo Ivi INFECTIONS IN THE IMMUNOCOMPROMISED HOST

















- Slides: 49

prof Ivo Ivić INFECTIONS IN THE IMMUNOCOMPROMISED HOST

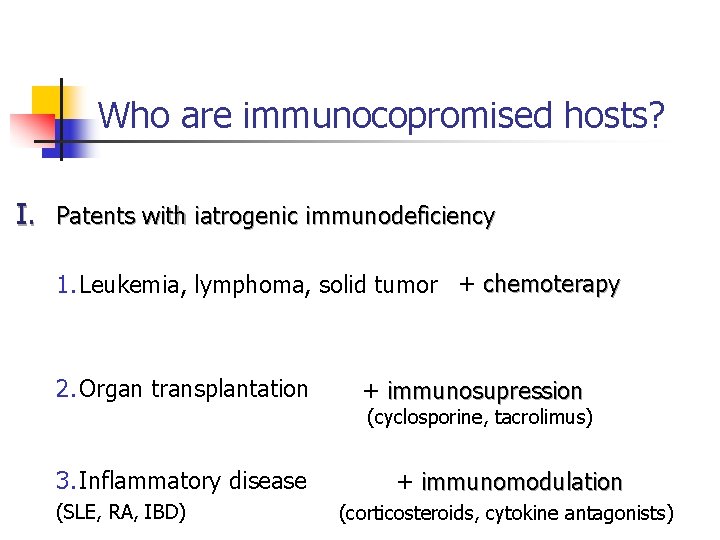
Who are immunocopromised hosts? I. Patents with iatrogenic immunodeficiency 1. Leukemia, lymphoma, solid tumor + chemoterapy 2. Organ transplantation + immunosupression (cyclosporine, tacrolimus) 3. Inflammatory disease (SLE, RA, IBD) + immunomodulation (corticosteroids, cytokine antagonists)

II. Patinets with genetic (hereditay) immunodeficiency of: n Immunoglobulins, neutrophils, complement III. Asplenic (anatomic or functional) patients: n Opsonic antibodies deficiency

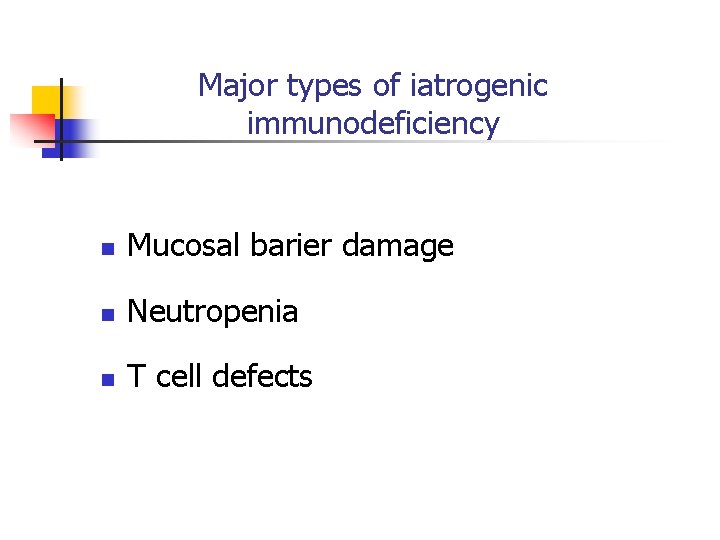
Major types of iatrogenic immunodeficiency n Mucosal barier damage n Neutropenia n T cell defects

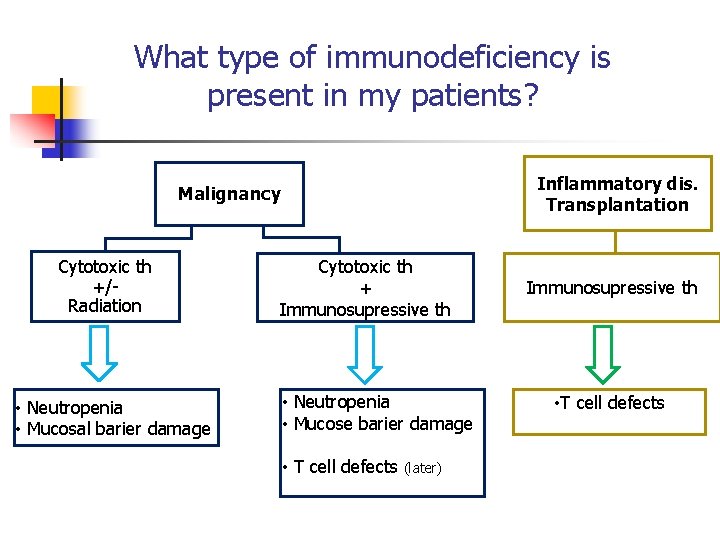
What type of immunodeficiency is present in my patients? Inflammatory dis. Transplantation Malignancy Cytotoxic th +/Radiation • Neutropenia • Mucosal barier damage Cytotoxic th + Immunosupressive th • Neutropenia • Mucose barier damage • T cell defects (later) Immunosupressive th • T cell defects

Patient with neutropenia and mucositis

Neutrophils: n n phagocytize and kill bacteria passing through mucosal membranes Neutropenia = neutrophil count <500/mm 3 Agranulocytosis = neutrophil count <200/mm 3 Hihg risk for serious bacterial infection: n If there is neutropenia for >10 days and/or n If there is agranulocytosis

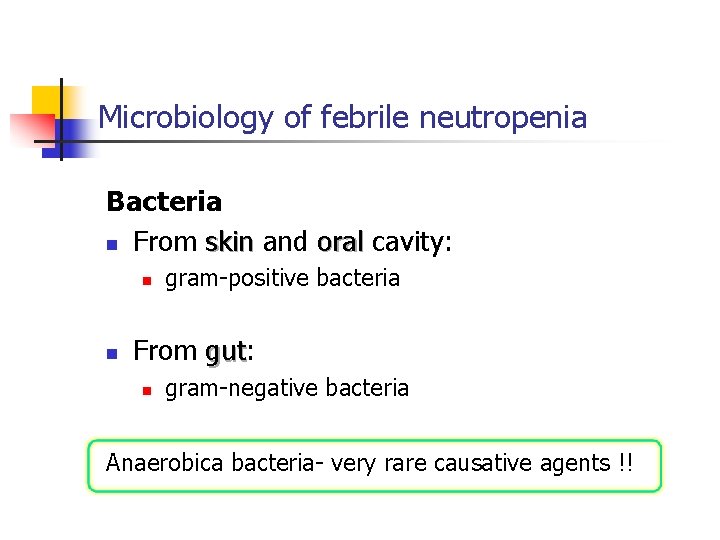
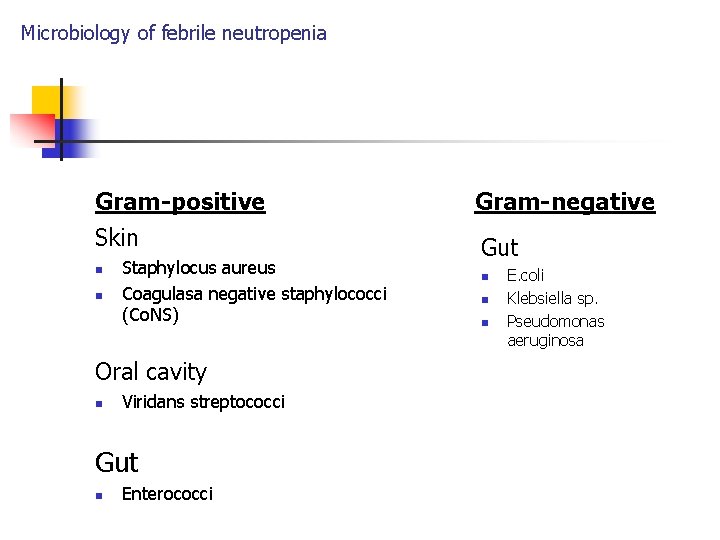
Microbiology of febrile neutropenia Bacteria n From skin and oral cavity: n n gram-positive bacteria From gut: gut n gram-negative bacteria Anaerobica bacteria- very rare causative agents !!

Microbiology of febrile neutropenia Gram-positive Gram-negative Skin Gut n n Staphylocus aureus Coagulasa negative staphylococci (Co. NS) Oral cavity n Viridans streptococci Gut n Enterococci n n n E. coli Klebsiella sp. Pseudomonas aeruginosa

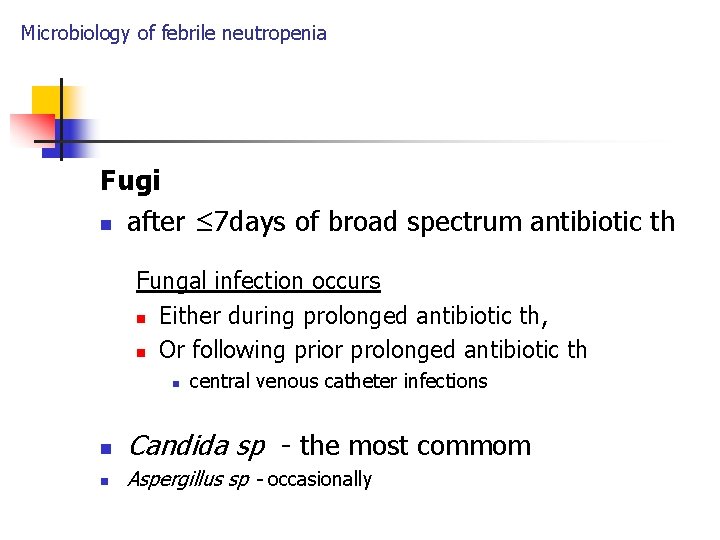
Microbiology of febrile neutropenia Fugi n after ≤ 7 days of broad spectrum antibiotic th Fungal infection occurs n Either during prolonged antibiotic th, n Or following prior prolonged antibiotic th n central venous catheter infections n Candida sp - the most commom n Aspergillus sp - occasionally

Patients with T cell supression

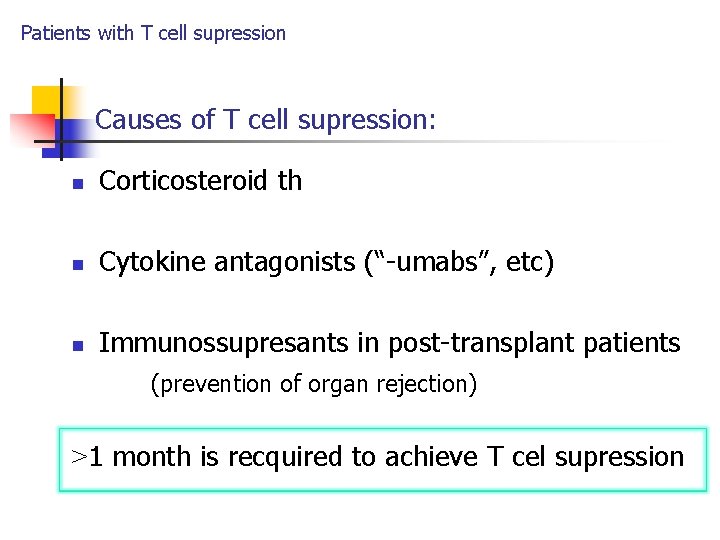
Patients with T cell supression Causes of T cell supression: n Corticosteroid th n Cytokine antagonists (“-umabs”, etc) n Immunossupresants in post-transplant patients (prevention of organ rejection) >1 month is recquired to achieve T cel supression

Patients with T cell supression Patients with solid organ transplantation

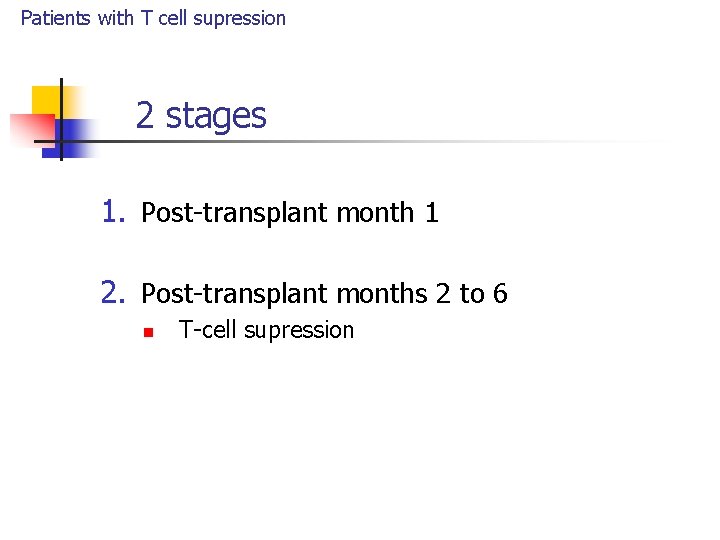
Patients with T cell supression 2 stages 1. Post-transplant month 1 2. Post-transplant months 2 to 6 n T-cell supression

Patients with T cell supression Microbiology of patients with solid organ transplantation 1. Post-transplant month 1 Hospital-acquired pathogens as in other patients: n frequently antimicrobial-resistant species n n n Pseudomonas, other gram-negative bacteria MRSA, VRE Azole-resistant Candida Legionella sp. in patients with penumonia Cl. difficile in patients with postantibotic diarrhoea

Patients with T cell supression 2. Post-transplant months 2 to 6 Bacteria n Mycobacterium tbc: : latent miliary tb n Listeria monocytogenes: meningitis n Nocardia sp. : cavitary/nodular pneumonia n L. pneumophila: penumonia

Patients with T cell supression (post-transplant month 2 -6) Fugi serious illness - difficult to diagnose n Cryptococcus neoformans – the most frequent n pulmonary, CNS Aspergilus sp. Fusarium sp. , etc. n n Candida ? n No!! No – controlled by neutrophils

Patients with T cell supression (post-transplant month 2 -6) Viruses Loos of cell mediated immunity reactivation of: n n patient’s latent viral infection donor’s latent viral infection (organ, blood transfusion) n CMV- most common n especialy if: recipent neg. /donor poz. for CMV Ab n EBV- less common: lymphoproliferative sy. n Rarely: HSV, VZV, HHV-6, HBV, HCV

Patients with T cell supression (post-transplant month 2 -6) Other pathogens n Pneumocystis jiroveci n n Toxoplasmosis n n interstital pneumonia, dyspnea, LDH brain abscess, encephalitis Strongyloides n eosinophilia

Patients with bone marrow transplantation

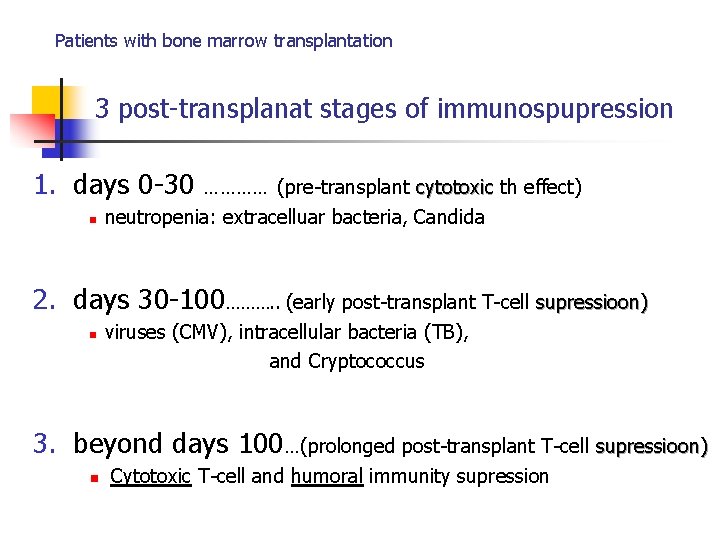
Patients with bone marrow transplantation 3 post-transplanat stages of immunospupression 1. days 0 -30 n ………… (pre-transplant cytotoxic th effect) neutropenia: extracelluar bacteria, Candida 2. days 30 -100………. . (early post-transplant T-cell supressioon) n viruses (CMV), intracellular bacteria (TB), and Cryptococcus 3. beyond days 100…(prolonged post-transplant T-cell supressioon) n Cytotoxic T-cell and humoral immunity supression

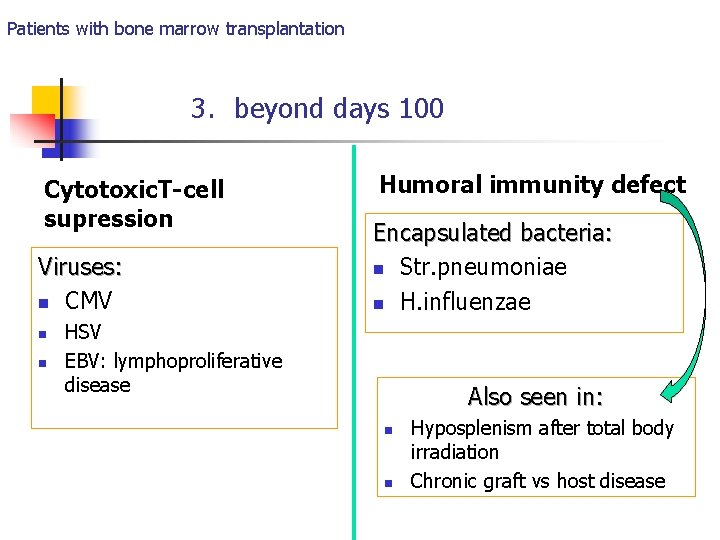
Patients with bone marrow transplantation 3. beyond days 100 Cytotoxic. T-cell supression Viruses: n CMV n n Humoral immunity defect Encapsulated bacteria: n Str. pneumoniae n H. influenzae HSV EBV: lymphoproliferative disease Also seen in: n n Hyposplenism after total body irradiation Chronic graft vs host disease

Approach to febrile neutropenic patient

Approach to febrilne neutropenia If fever >38, 30 C n Initial workup n Administration of antibiotic within 60 min. Warning! In early stage of infection: n n fever may bee the only sign of infection specific organ symptoms - frequently absent !

Approach to febrilne neutropenia Initial workup n Thorough physical examination (site of infection? ) n Blood culture: peripheril vein + central line n n Cultures from other affected sites (urin, pus, sinovial fluid, etc. ) Biopsy and culture of any new skin lesions Blod tests: ESR, WBC + differential, Plt, BUN, kreatinin, AST, ALT, bilirubin Chest X-ray

Approach to febrilne neutropenia Initial antibiotic therapy n Highly severe disease- intravenous th: n n Monotherapy: cefepime, imipenem, piperacilin-tazobactam or Dual therapy: one of above + aminoglycosides or quinolones Addition of vancomycin? , only: - in patients with proven S. aureus colonisation if gram-positive cocci grow in blood culture before sensitivity testing empiricaly- only patients with cardiovascular compromise

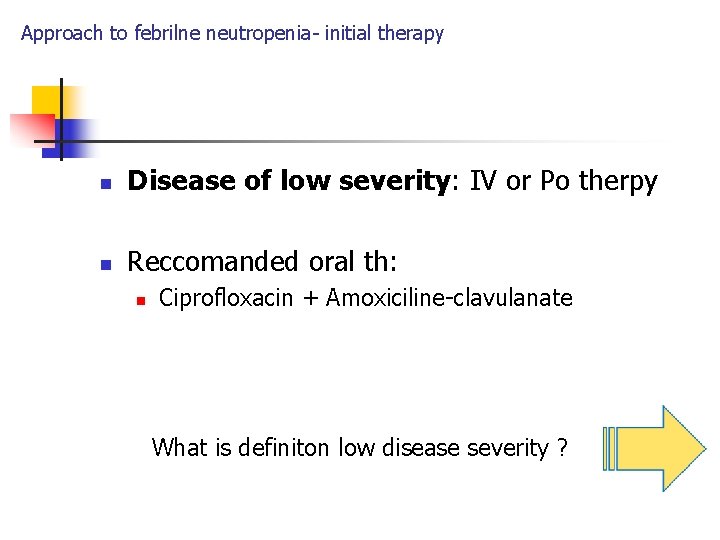
Approach to febrilne neutropenia- initial therapy n Disease of low severity: IV or Po therpy n Reccomanded oral th: n Ciprofloxacin + Amoxiciline-clavulanate What is definiton low disease severity ?

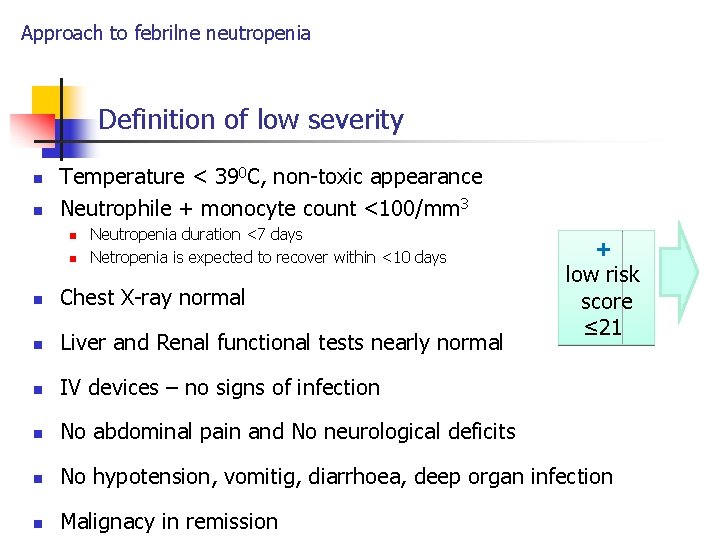
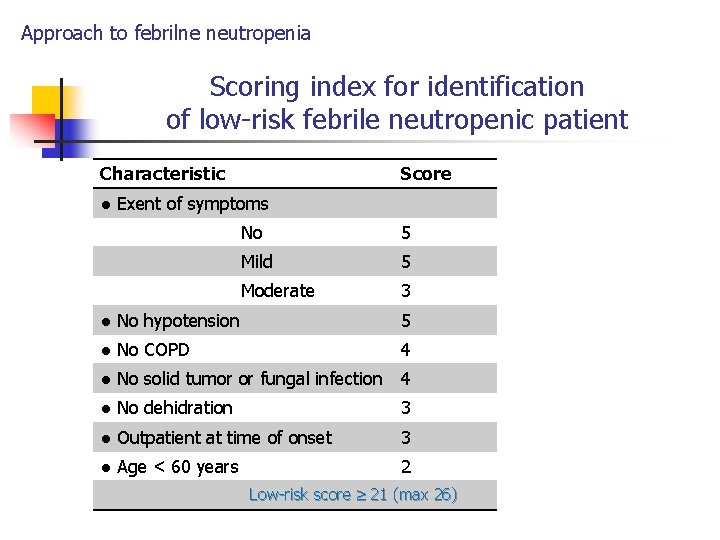
Approach to febrilne neutropenia Definition of low severity n n Temperature < 390 C, non-toxic appearance Neutrophile + monocyte count <100/mm 3 n n Neutropenia duration <7 days Netropenia is expected to recover within <10 days + low risk score ≤ 21 n Chest X-ray normal n Liver and Renal functional tests nearly normal n IV devices – no signs of infection n No abdominal pain and No neurological deficits n No hypotension, vomitig, diarrhoea, deep organ infection n Malignacy in remission

Approach to febrilne neutropenia Scoring index for identification of low-risk febrile neutropenic patient Characteristic Score ● Exent of symptoms No 5 Mild 5 Moderate 3 ● No hypotension 5 ● No COPD 4 ● No solid tumor or fungal infection 4 ● No dehidration 3 ● Outpatient at time of onset 3 ● Age < 60 years 2 Low-risk score 21 (max 26)

Approach to febrilne neutropenia Therapeutic goals n Drop of fever within 3 -5 days and n Neutrophil count 500/mm 3 Duration fo therapy: not less than 7 days Minimum requirements for cessation of therapy: • 2 days wihout fever, and • 2 days of neutrophil count >500/mm 3

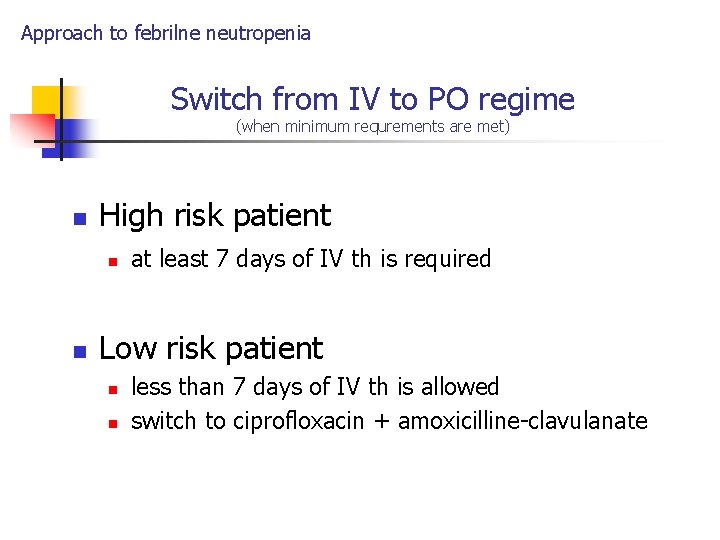
Approach to febrilne neutropenia Switch from IV to PO regime (when minimum requrements are met) n High risk patient n n at least 7 days of IV th is required Low risk patient n n less than 7 days of IV th is allowed switch to ciprofloxacin + amoxicilline-clavulanate

Approach to febrilne neutropenia Other circumstances

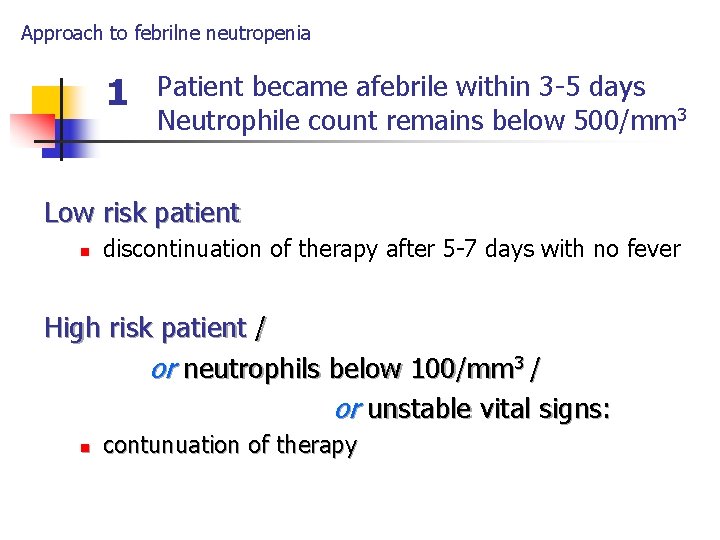
Approach to febrilne neutropenia 1 Patient became afebrile within 3 -5 days Neutrophile count remains below 500/mm 3 Low risk patient n discontinuation of therapy after 5 -7 days with no fever High risk patient / or neutrophils below 100/mm 3 / or unstable vital signs: n contunuation of therapy

Approach to febrilne neutropenia 2 a The peristently febrile patient n Neutrophile count >500/mm 3 for 4 -5 days n n Discontiune antibiotics Reassess the patient

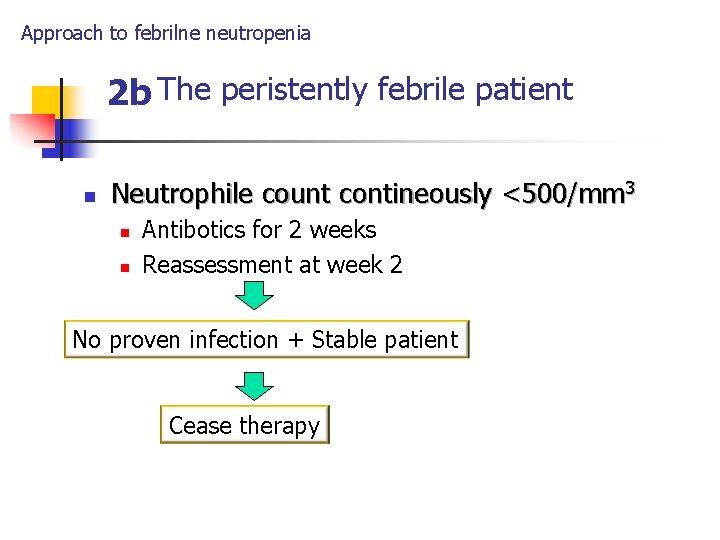
Approach to febrilne neutropenia 2 b The peristently febrile patient n Neutrophile count contineously <500/mm 3 n n Antibotics for 2 weeks Reassessment at week 2 No proven infection + Stable patient Cease therapy

Approach to febrile patient with depressed cell-mediated immunity (non-neutropenic patient)

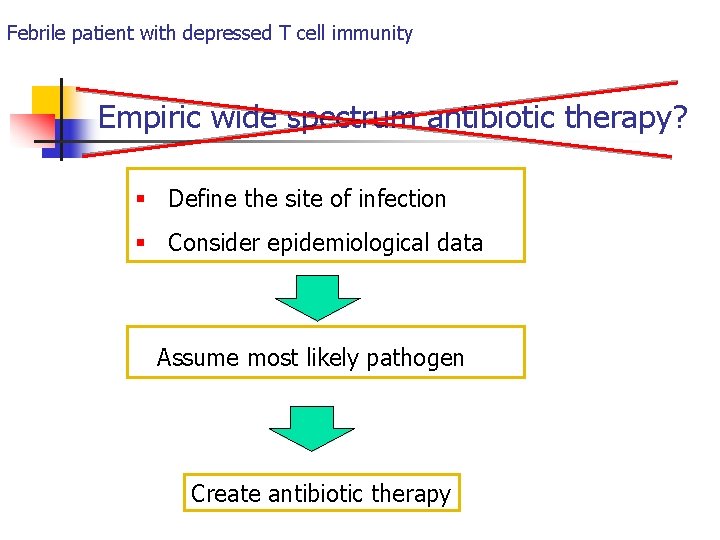
Febrile patient with depressed T cell immunity Empiric wide spectrum antibiotic therapy? § Define the site of infection § Consider epidemiological data Assume most likely pathogen Create antibiotic therapy

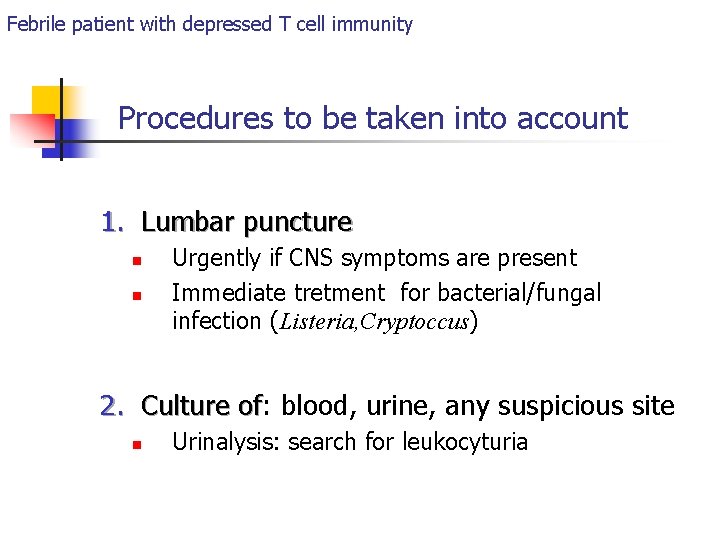
Febrile patient with depressed T cell immunity Procedures to be taken into account 1. Lumbar puncture n n Urgently if CNS symptoms are present Immediate tretment for bacterial/fungal infection (Listeria, Cryptoccus) 2. Culture of: of blood, urine, any suspicious site n Urinalysis: search for leukocyturia

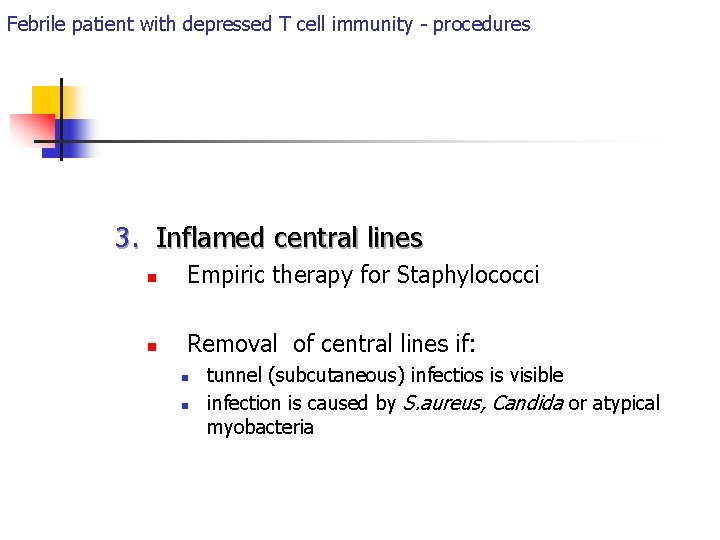
Febrile patient with depressed T cell immunity - procedures 3. Inflamed central lines n Empiric therapy for Staphylococci n Removal of central lines if: n n tunnel (subcutaneous) infectios is visible infection is caused by S. aureus, Candida or atypical myobacteria

Febrile patient with depressed T cell immunity - procedures 4. Abnormal chest X-ray! X-ray n Prior to therapy: n In patient with sputum production: n n n sputum cultures (bacteria, mycobacteria, fungi) stainings (Gram, acid fast, silver) In patient with no sputum n sampling by bronhoscopy

Febrile patient with depressed T cell immunity - procedures 5. Patient is febrile + no infection is confirmed (all previous test are negative) Do not waste time Consult infectologist

Febrile patient with depressed T cell immunity About corticosteroids ! n The most frequently used immunosupression n They also supress inflammatory response n low grade fever may indiciate seroius infection

Prevention of infections in patients with depressed cell-mediated immunity (transplatant patients)

Prevention of bacterial infections n S. pneumoniae (pneumonia and sinusitis) n only if Ig. G level <4 g/L n intravenous Ig. G (IVIG)

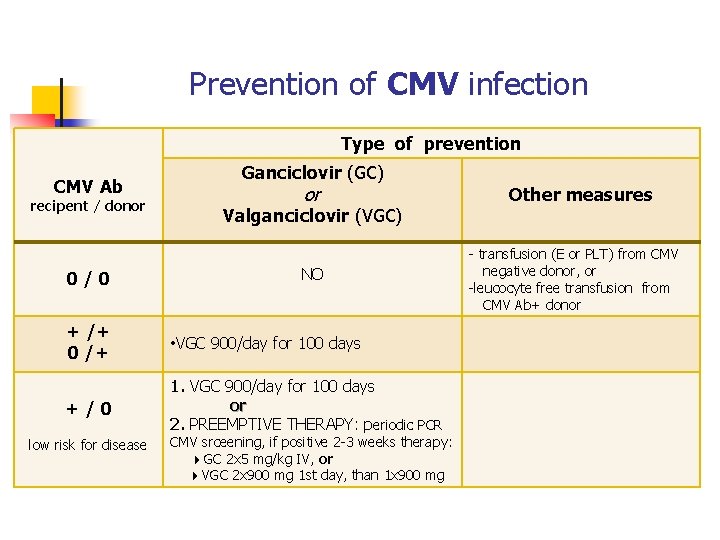
Prevention of CMV infection Type of prevention CMV Ab recipent / donor 0/0 Ganciclovir (GC) or Valganciclovir (VGC) NO + /+ 0 /+ • VGC 900/day for 100 days +/0 1. VGC 900/day for 100 days or 2. PREEMPTIVE THERAPY: periodic PCR low risk for disease CMV srceening, if positive 2 -3 weeks therapy: GC 2 x 5 mg/kg IV, or VGC 2 x 900 mg 1 st day, than 1 x 900 mg Other measures - transfusion (E or PLT) from CMV negative donor, or -leucocyte free transfusion from CMV Ab+ donor

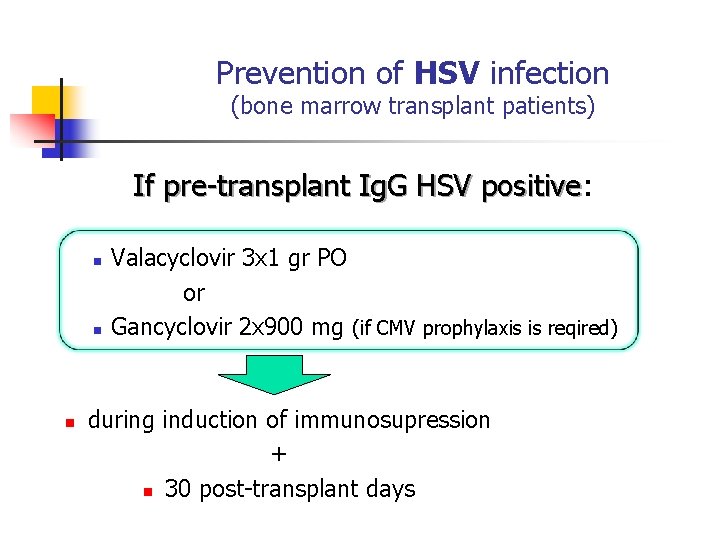
Prevention of HSV infection (bone marrow transplant patients) If pre-transplant Ig. G HSV positive: positive n n n Valacyclovir 3 x 1 gr PO or Gancyclovir 2 x 900 mg (if CMV prophylaxis is reqired) during induction of immunosupression + n 30 post-transplant days

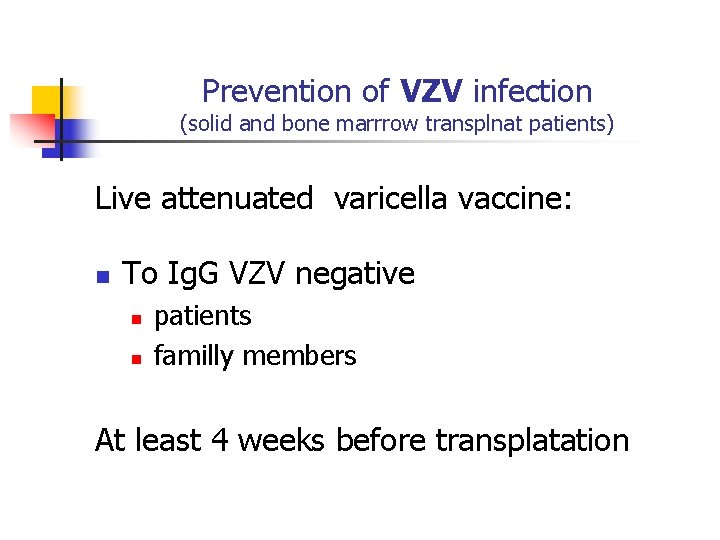
Prevention of VZV infection (solid and bone marrrow transplnat patients) Live attenuated varicella vaccine: n To Ig. G VZV negative n n patients familly members At least 4 weeks before transplatation

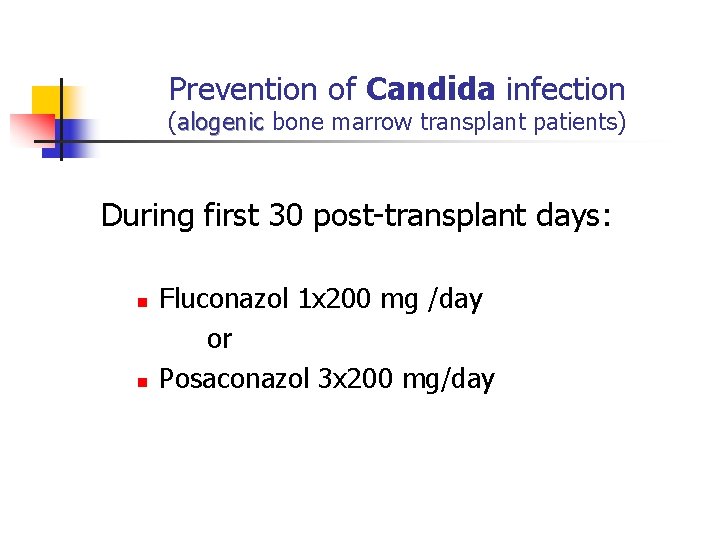
Prevention of Candida infection (alogenic bone marrow transplant patients) During first 30 post-transplant days: n n Fluconazol 1 x 200 mg /day or Posaconazol 3 x 200 mg/day

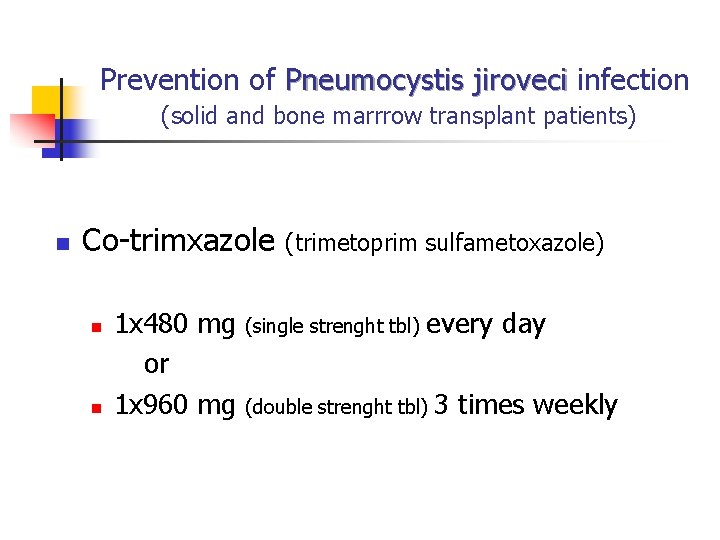
Prevention of Pneumocystis jiroveci infection (solid and bone marrrow transplant patients) n Co-trimxazole n n 1 x 480 mg or 1 x 960 mg (trimetoprim sulfametoxazole) (single strenght tbl) every day (double strenght tbl) 3 times weekly