PRODUCTION OF TITANIUM FROM WASTE SLAG Samuel MartinTreceno

![BACKGROUND PRODUCTION OF TITANIUM Kroll [1] process FFC [2] process Precursor Ti. Cl 4 BACKGROUND PRODUCTION OF TITANIUM Kroll [1] process FFC [2] process Precursor Ti. Cl 4](https://slidetodoc.com/presentation_image/0824ed96dc44e2e783d6bfc2a0503281/image-4.jpg)



- Slides: 15

PRODUCTION OF TITANIUM FROM WASTE SLAG Samuel Martin-Treceno 1, Thomas Hughes 1, Catherine Bishop 2, Ian Brown 3, Yaodong Jia 3, Aaron Marshall 1, Matthew Watson 1 1 Department of Chemical and Process Engineering, University of Canterbury, Christchurch, New Zealand 2 Department of Mechanical Engineering, University of Canterbury, Christchurch, New Zealand 3 Callaghan Innovation, Lower Hutt, New Zealand https: //www. nzsteel. co. nz/products/aggregates/ 1

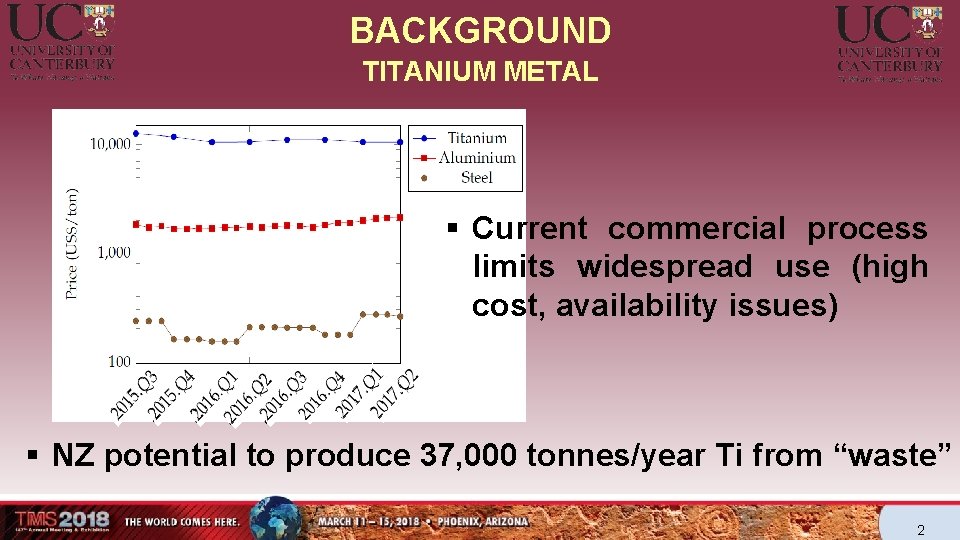
BACKGROUND TITANIUM METAL § Current commercial process limits widespread use (high cost, availability issues) § NZ potential to produce 37, 000 tonnes/year Ti from “waste” 2

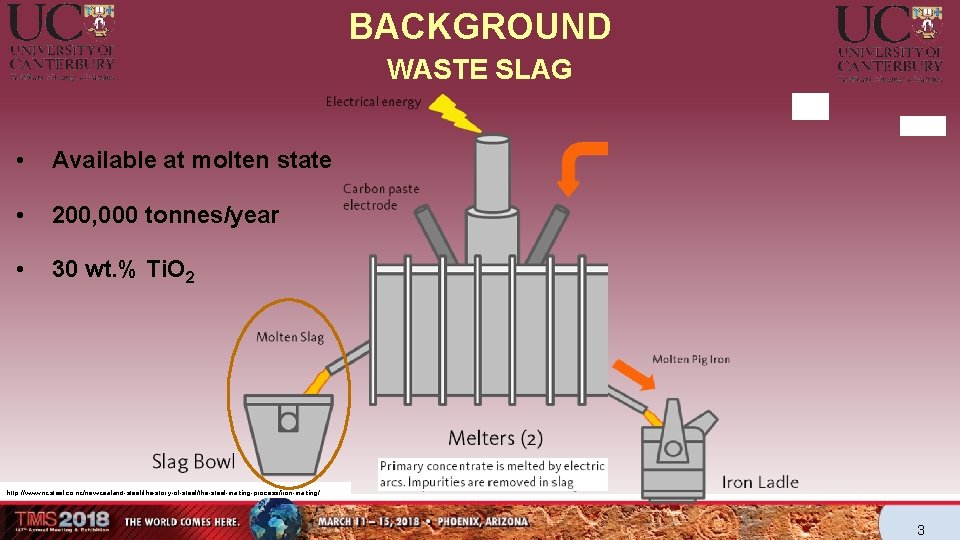
BACKGROUND WASTE SLAG • Available at molten state • 200, 000 tonnes/year • 30 wt. % Ti. O 2 http: //www. nzsteel. co. nz/new-zealand-steel/the-story-of-steel/the-steel-making-process/iron-making/ 3
![BACKGROUND PRODUCTION OF TITANIUM Kroll 1 process FFC 2 process Precursor Ti Cl 4 BACKGROUND PRODUCTION OF TITANIUM Kroll [1] process FFC [2] process Precursor Ti. Cl 4](https://slidetodoc.com/presentation_image/0824ed96dc44e2e783d6bfc2a0503281/image-4.jpg)
BACKGROUND PRODUCTION OF TITANIUM Kroll [1] process FFC [2] process Precursor Ti. Cl 4 Ti. O 2 Ti-bearing ore Year 1940 2000 ? Reductant Mg e- e- [1] W. J. Kroll, Trans. Electrochem. Soc. , 1940, 78, 35– 47. [2] G. Z. Chen, D. J. Fray and T. W. Farthing: Nature, 2000, 407, 361– 364. 4

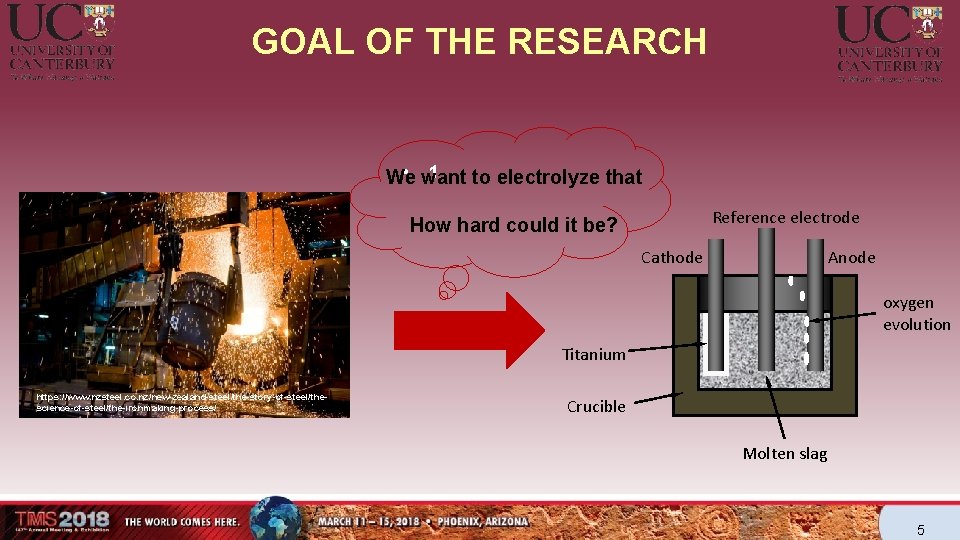
GOAL OF THE RESEARCH 1 • want We to electrolyze that Reference electrode How hard could it be? Anode Cathode oxygen evolution Titanium https: //www. nzsteel. co. nz/new-zealand-steel/the-story-of-steel/thescience-of-steel/the-ironmaking-process/ Crucible Molten slag 5

TECHNICAL CHALLENGES AT 1800 K Oxidation Before After Crucible failure Before After Circumferential crack The unofficial Laws of High Temperatures states: 1. ”At high temperatures everything reacts with everything else” 2. “They react bloody quickly and it gets exponentially worse as the temperature increases “ - Professor K. C. Mills 6

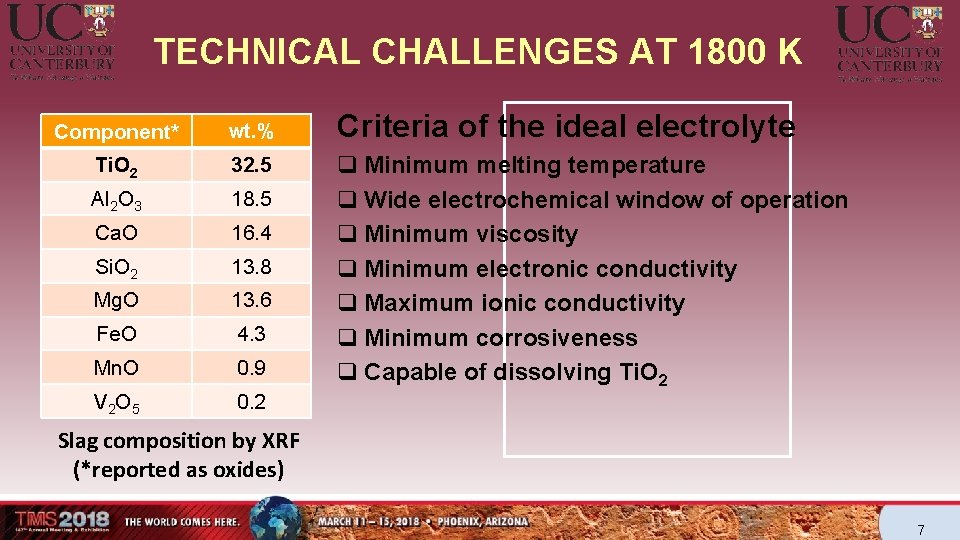
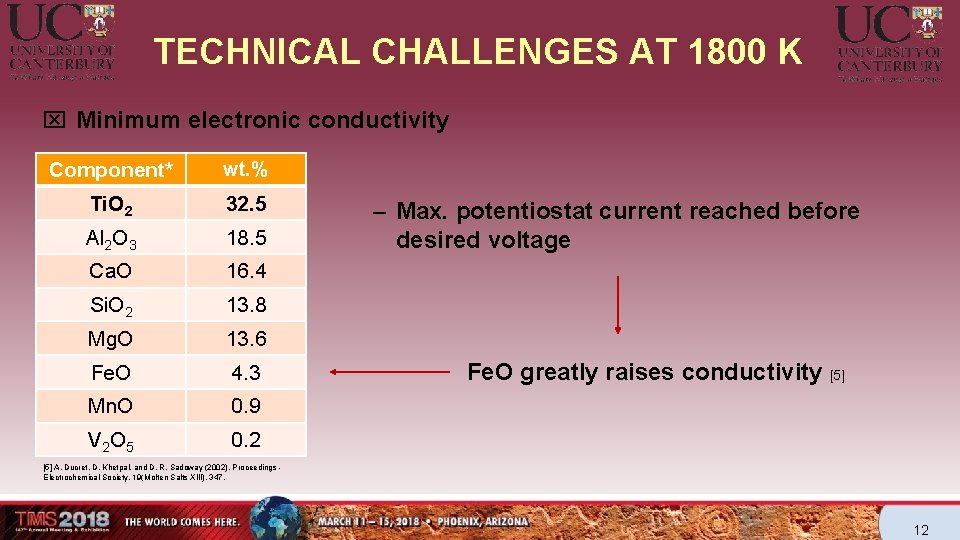
TECHNICAL CHALLENGES AT 1800 K Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 Criteria of the ideal electrolyte q Minimum melting temperature q Wide electrochemical window of operation q Minimum viscosity q Minimum electronic conductivity q Maximum ionic conductivity q Minimum corrosiveness q Capable of dissolving Ti. O 2 Slag composition by XRF (*reported as oxides) 7

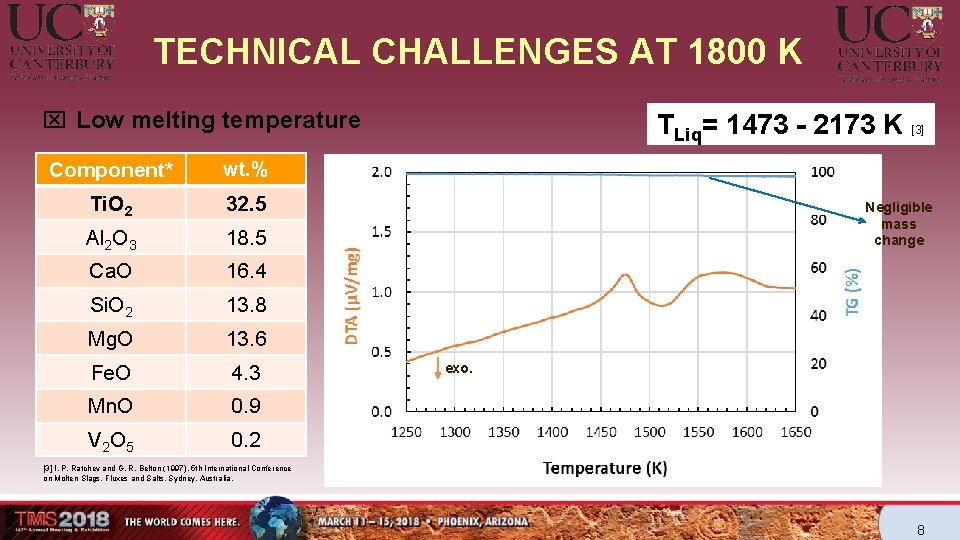
TECHNICAL CHALLENGES AT 1800 K x Low melting temperature Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 TLiq= 1473 - 2173 K [3] Negligible mass change exo. [3] I. P. Ratchev and G. R. Belton (1997). 5 th International Conference on Molten Slags, Fluxes and Salts, Sydney, Australia. 8

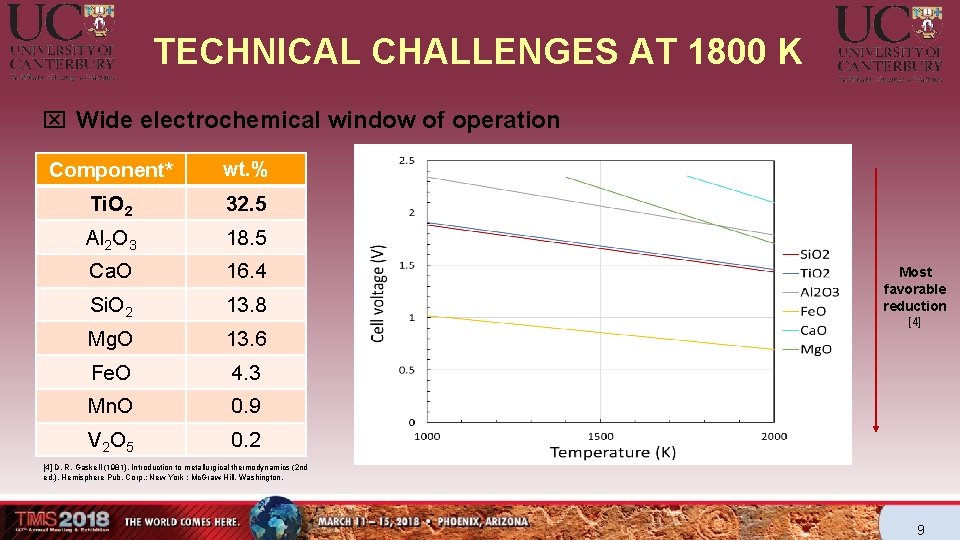
TECHNICAL CHALLENGES AT 1800 K x Wide electrochemical window of operation Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 Most favorable reduction [4] D. R. Gaskell (1981). Introduction to metallurgical thermodynamics (2 nd ed. ). Hemisphere Pub. Corp. ; New York : Mc. Graw-Hill, Washington. 9

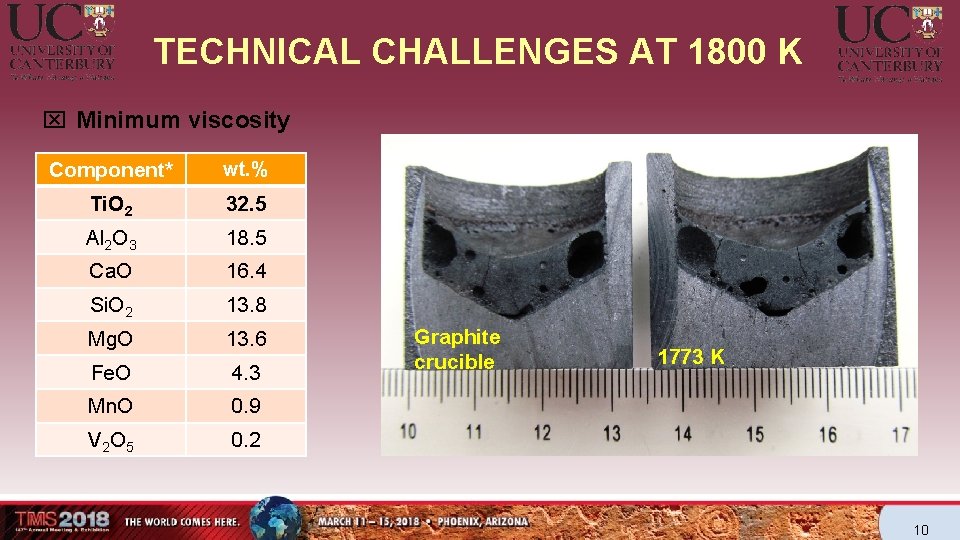
TECHNICAL CHALLENGES AT 1800 K x Minimum viscosity Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 Graphite crucible 1773 K 10

TECHNICAL CHALLENGES AT 1800 K x Minimum corrosiveness Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 – Ceramic soluble in molten oxide electrolyte – Metal soluble in liquid metal product Single-use crucible + secondary container 11

TECHNICAL CHALLENGES AT 1800 K x Minimum electronic conductivity Component* wt. % Ti. O 2 32. 5 Al 2 O 3 18. 5 Ca. O 16. 4 Si. O 2 13. 8 Mg. O 13. 6 Fe. O 4. 3 Mn. O 0. 9 V 2 O 5 0. 2 – Max. potentiostat current reached before desired voltage Fe. O greatly raises conductivity [5] A. Ducret, D. Khetpal, and D. R. Sadoway (2002). Proceedings Electrochemical Society, 19(Molten Salts XIII), 347. 12

ATTEMPTS TO DATE Issues Fragility of the system Slag electrically conductive Data corruption due to - Furnace tube failure - Crucible failure: CTE mismatch upon cooling Iron droplets 1823 K Graphite crucible 13

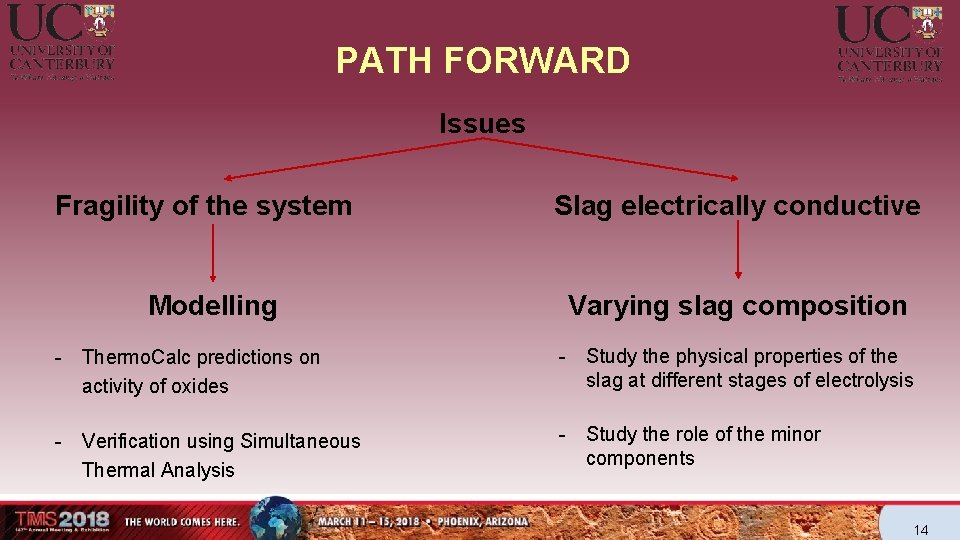
PATH FORWARD Issues Fragility of the system Modelling Slag electrically conductive Varying slag composition - Thermo. Calc predictions on activity of oxides - Study the physical properties of the slag at different stages of electrolysis - Verification using Simultaneous Thermal Analysis - Study the role of the minor components 14

ACKNOWLEDGMENTS THANK YOU FOR YOUR ATTENTION! 15