Production of Recombinant Proteins Protein Production in Prokaryotic
Production of Recombinant Proteins • Protein Production in Prokaryotic Hosts • Regulation of transcription • Increasing translation efficiency • Increasing protein stability • Increasing protein secretion • Facilitating protein purification • DNA integration into the host chromosome • Protein Production in Eukaryotic Hosts • Posttranslational modification of eukaryotic proteins • Eukaryotic expression systems (general features, yeast, baculovirus-insect cells, mammalian cells) • Protein Engineering • Directed mutagenesis • Random mutagenesis • Examples of protein engineering
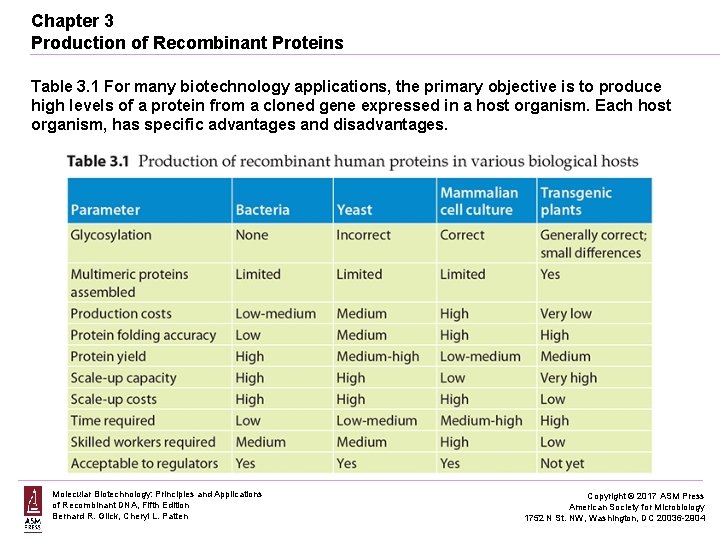
Chapter 3 Production of Recombinant Proteins Table 3. 1 For many biotechnology applications, the primary objective is to produce high levels of a protein from a cloned gene expressed in a host organism. Each host organism, has specific advantages and disadvantages. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
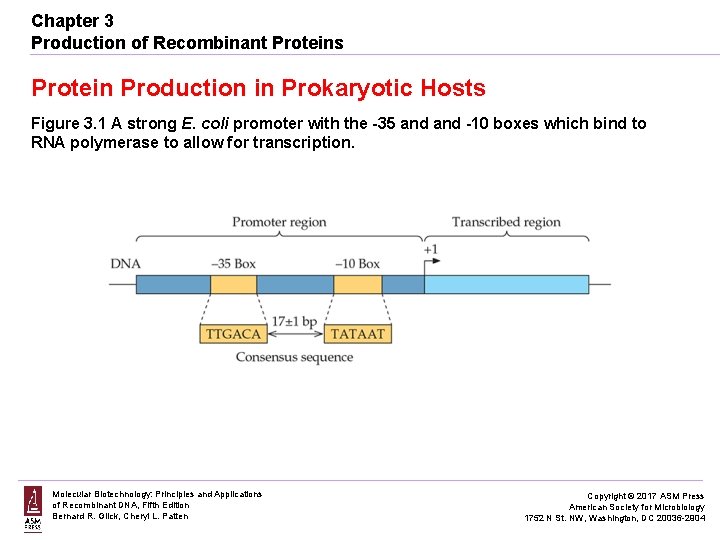
Chapter 3 Production of Recombinant Proteins Protein Production in Prokaryotic Hosts Figure 3. 1 A strong E. coli promoter with the -35 and -10 boxes which bind to RNA polymerase to allow for transcription. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
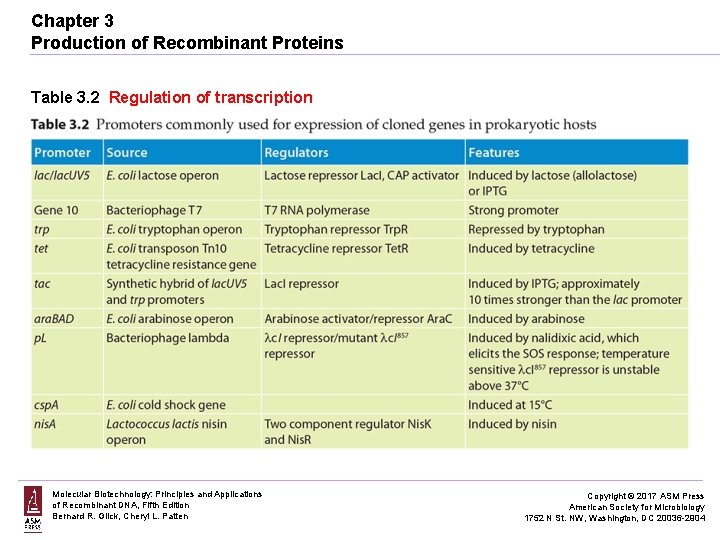
Chapter 3 Production of Recombinant Proteins Table 3. 2 Regulation of transcription Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
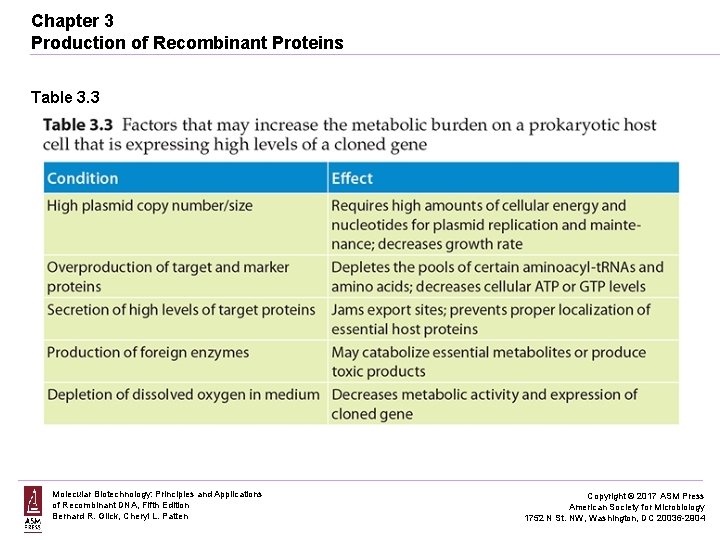
Chapter 3 Production of Recombinant Proteins Table 3. 3 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
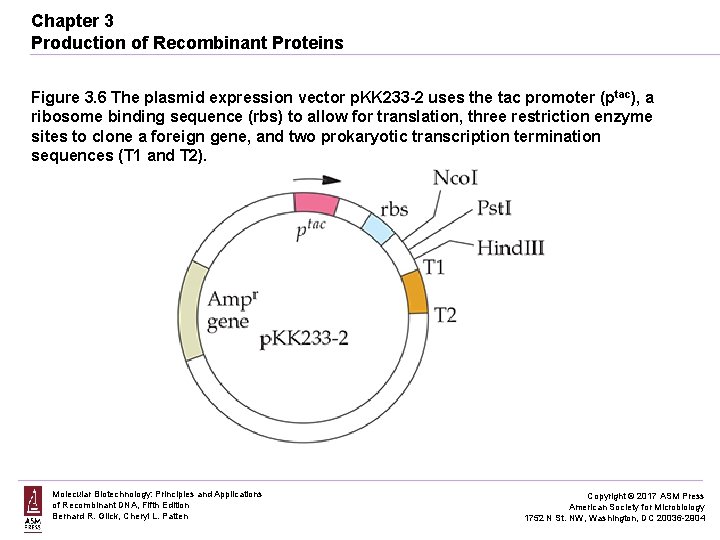
Chapter 3 Production of Recombinant Proteins Figure 3. 6 The plasmid expression vector p. KK 233 -2 uses the tac promoter (ptac), a ribosome binding sequence (rbs) to allow for translation, three restriction enzyme sites to clone a foreign gene, and two prokaryotic transcription termination sequences (T 1 and T 2). Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
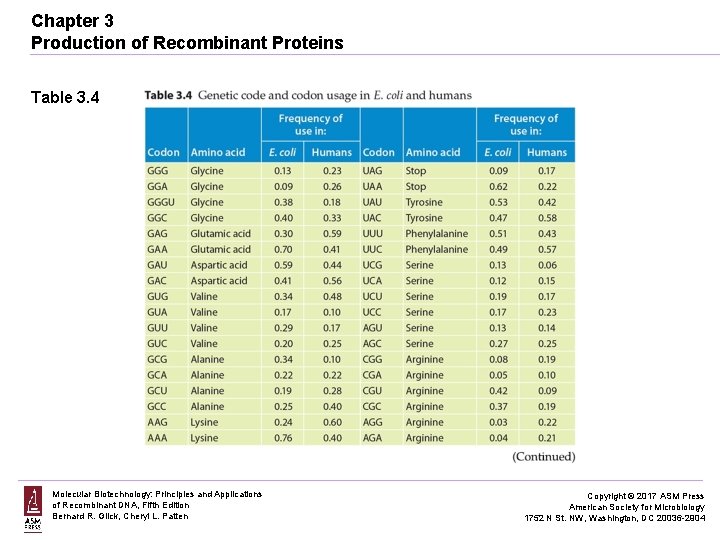
Chapter 3 Production of Recombinant Proteins Table 3. 4 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
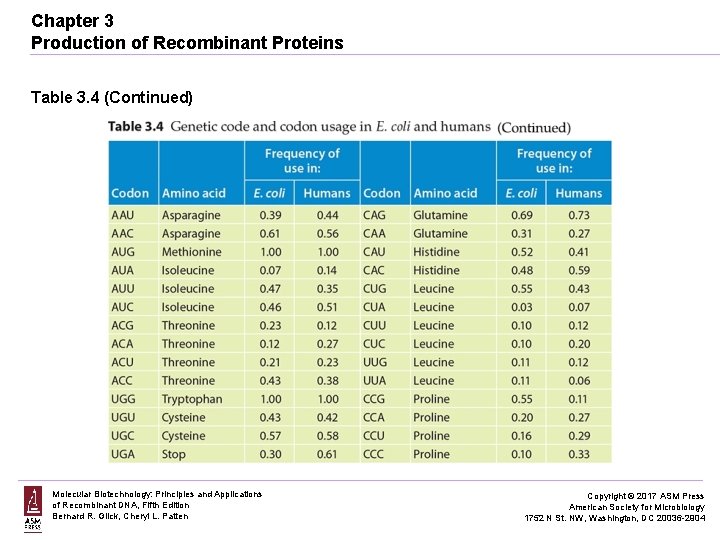
Chapter 3 Production of Recombinant Proteins Table 3. 4 (Continued) Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
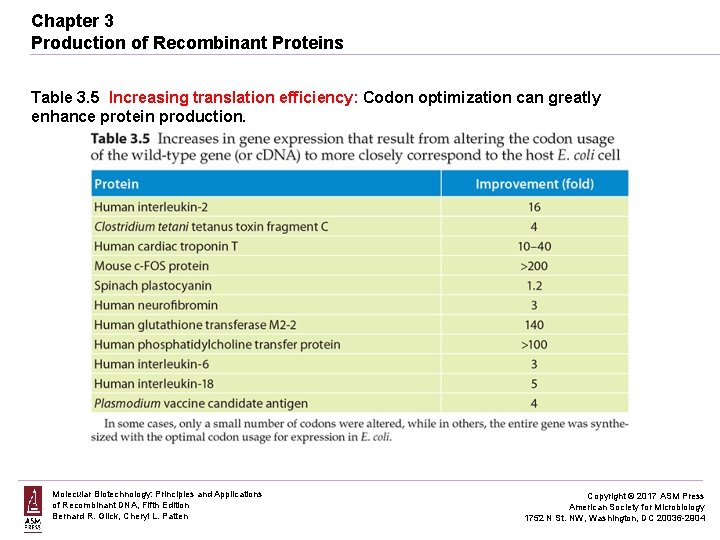
Chapter 3 Production of Recombinant Proteins Table 3. 5 Increasing translation efficiency: Codon optimization can greatly enhance protein production. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904

Increasing Protein Stability • By facilitating protein folding (e. g. , low temperature or coexpress with genes encoding proteins that function in proper protein folding such as chaperones) • By decreasing protein degradation (e. g. , add specific amino acids to the N-terminus to reduce protease digestion or express the protein as a fusion protein) Figure 3. 9

Chapter 3 Production of Recombinant Proteins Increasing Protein Secretion into the periplasm by adding a bacterial signal peptide sequence to the N-terminus generally allows for easier protein purification and reduced protein degradation as there are fewer proteins and proteases in the extracellular environment. Figure 3. 10 Protein secretion in bacteria. (A) Secretion pathway in Gram-negative bacteria like E. coli. B. Protein secretion in Gram-positive bacteria such as Lactococcus lactis. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
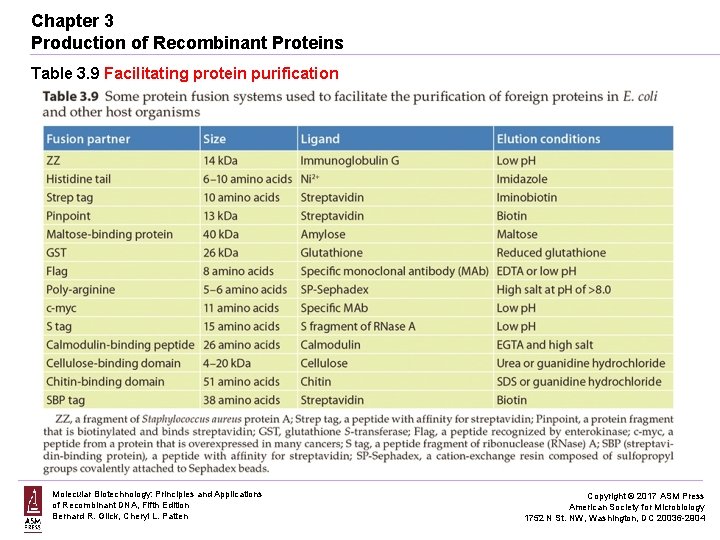
Chapter 3 Production of Recombinant Proteins Table 3. 9 Facilitating protein purification Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
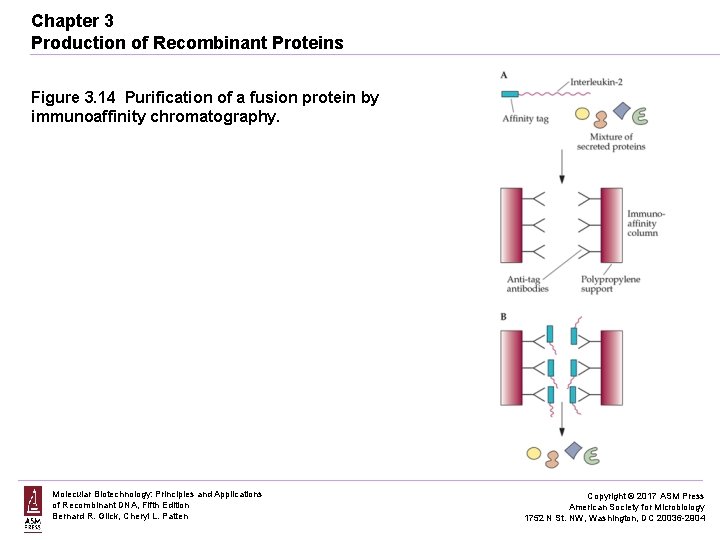
Chapter 3 Production of Recombinant Proteins Figure 3. 14 Purification of a fusion protein by immunoaffinity chromatography. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
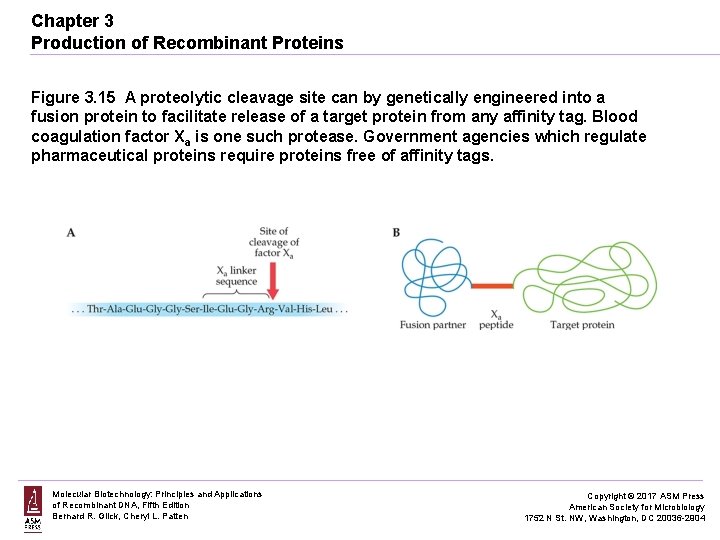
Chapter 3 Production of Recombinant Proteins Figure 3. 15 A proteolytic cleavage site can by genetically engineered into a fusion protein to facilitate release of a target protein from any affinity tag. Blood coagulation factor Xa is one such protease. Government agencies which regulate pharmaceutical proteins require proteins free of affinity tags. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
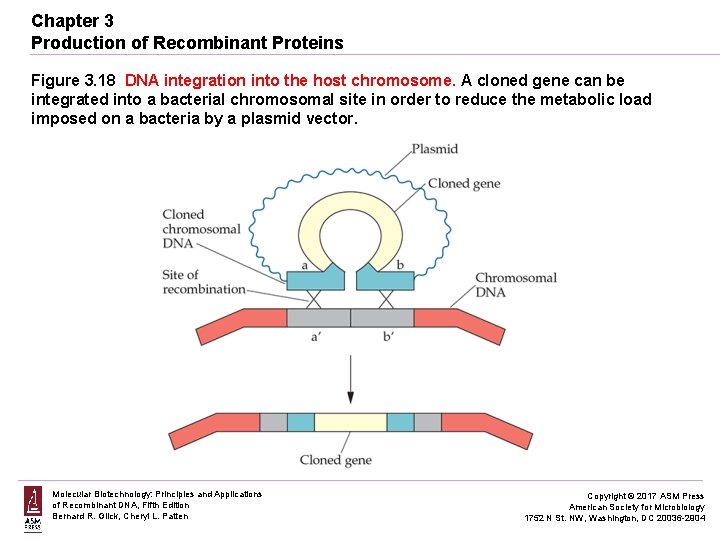
Chapter 3 Production of Recombinant Proteins Figure 3. 18 DNA integration into the host chromosome. A cloned gene can be integrated into a bacterial chromosomal site in order to reduce the metabolic load imposed on a bacteria by a plasmid vector. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
Protein Production in Eukaryotic Hosts • • Unlike prokaryotes, eukaryotes carry out a number of posttranslational modifications (PTMs) of proteins, which are often critical for proper protein function These PTMs include: • Glycosylation • Processing of propeptides • Phosphorylation • Acetylation • Methylation • Hydroxylation • Sulfation • Acylation • g-Carboxylation • Myristoylation (C 14 fatty acid addition) • Palmitoylation (C 16 fatty acid addition) • Disulfide bond formation
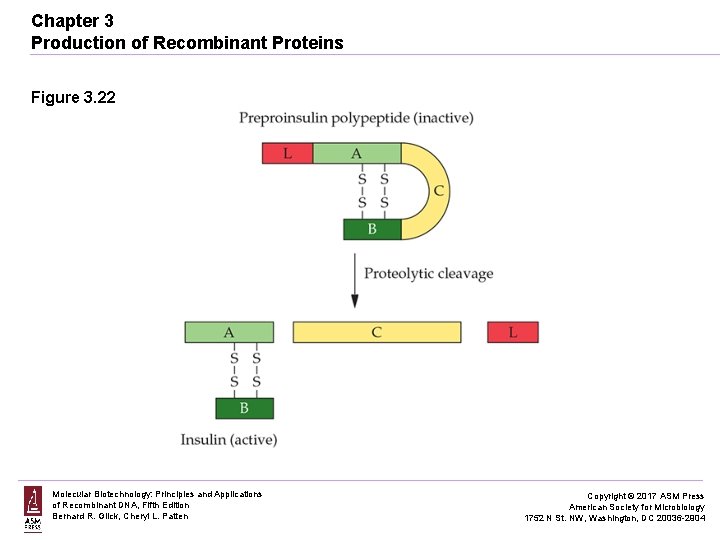
Chapter 3 Production of Recombinant Proteins Figure 3. 22 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
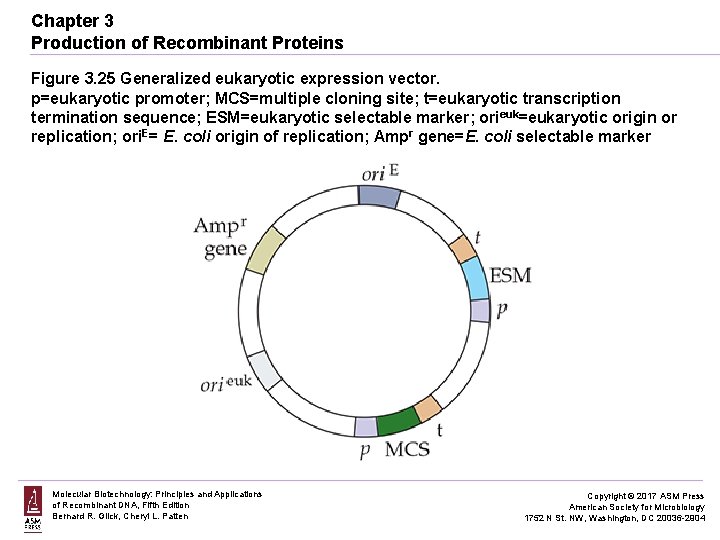
Chapter 3 Production of Recombinant Proteins Figure 3. 25 Generalized eukaryotic expression vector. p=eukaryotic promoter; MCS=multiple cloning site; t=eukaryotic transcription termination sequence; ESM=eukaryotic selectable marker; orieuk=eukaryotic origin or replication; ori. E= E. coli origin of replication; Ampr gene=E. coli selectable marker Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
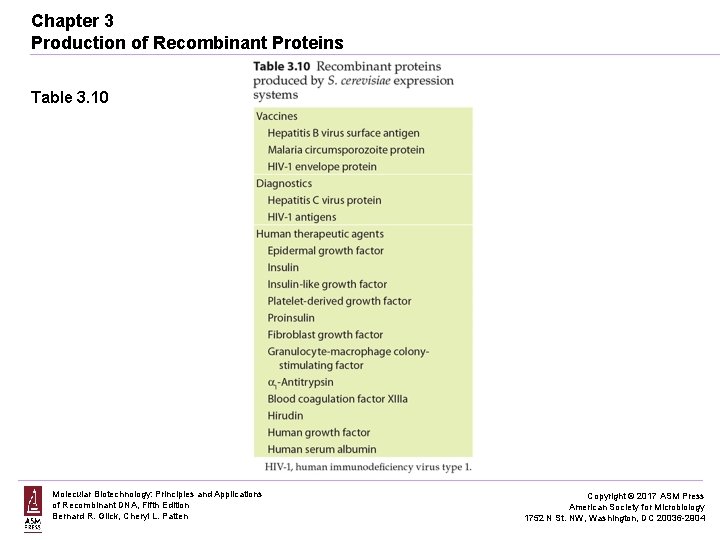
Chapter 3 Production of Recombinant Proteins Table 3. 10 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
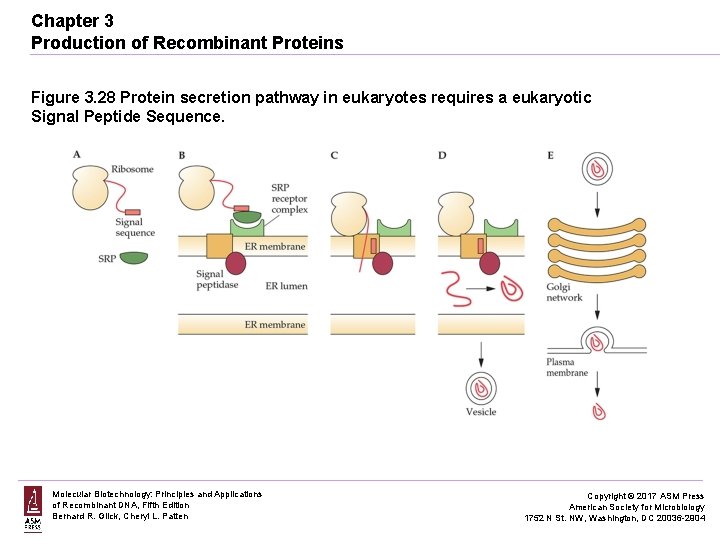
Chapter 3 Production of Recombinant Proteins Figure 3. 28 Protein secretion pathway in eukaryotes requires a eukaryotic Signal Peptide Sequence. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
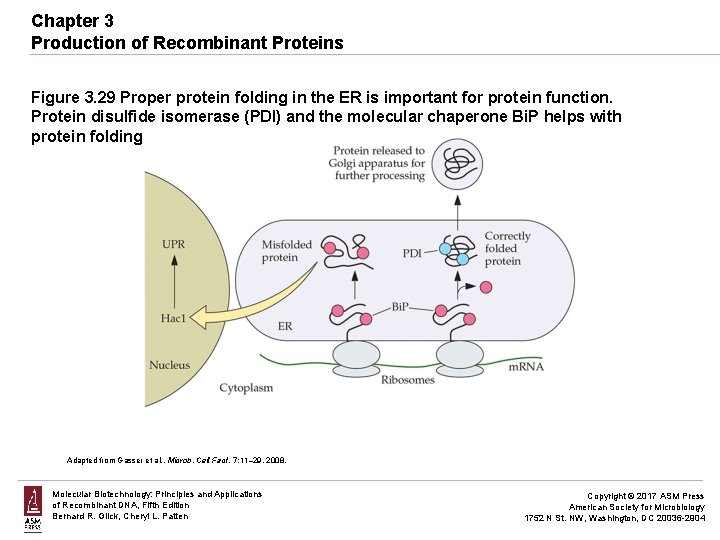
Chapter 3 Production of Recombinant Proteins Figure 3. 29 Proper protein folding in the ER is important for protein function. Protein disulfide isomerase (PDI) and the molecular chaperone Bi. P helps with protein folding. Adapted from Gasser et al. , Microb. Cell Fact. 7: 11– 29, 2008. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
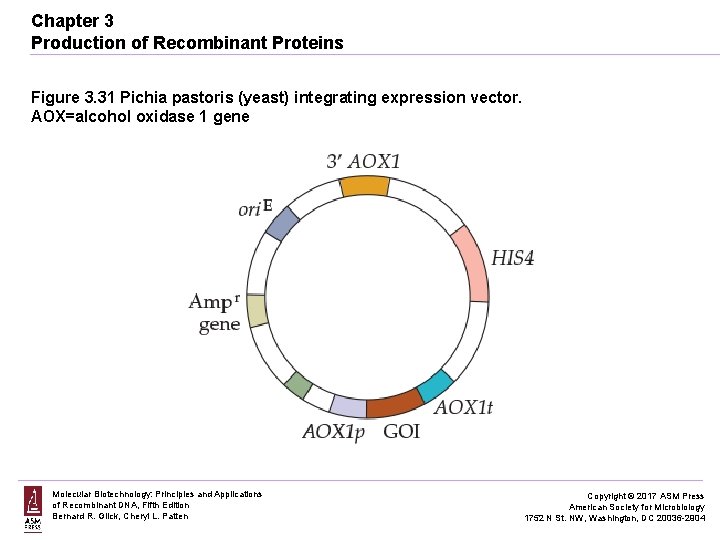
Chapter 3 Production of Recombinant Proteins Figure 3. 31 Pichia pastoris (yeast) integrating expression vector. AOX=alcohol oxidase 1 gene Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
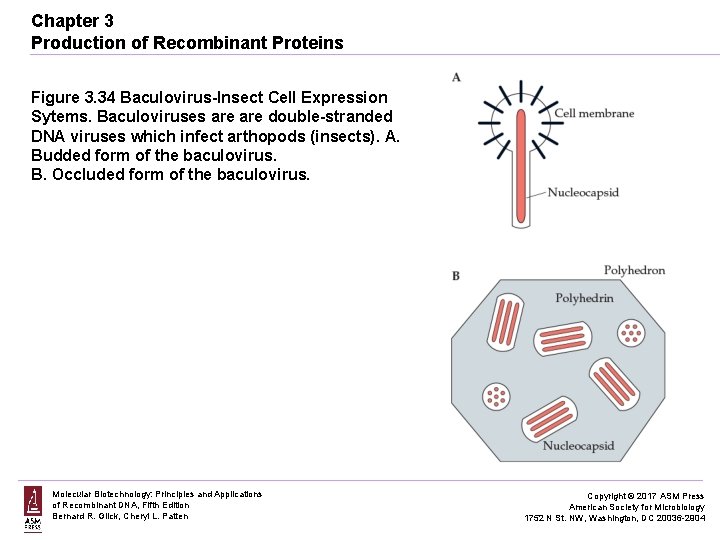
Chapter 3 Production of Recombinant Proteins Figure 3. 34 Baculovirus-Insect Cell Expression Sytems. Baculoviruses are double-stranded DNA viruses which infect arthopods (insects). A. Budded form of the baculovirus. B. Occluded form of the baculovirus. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
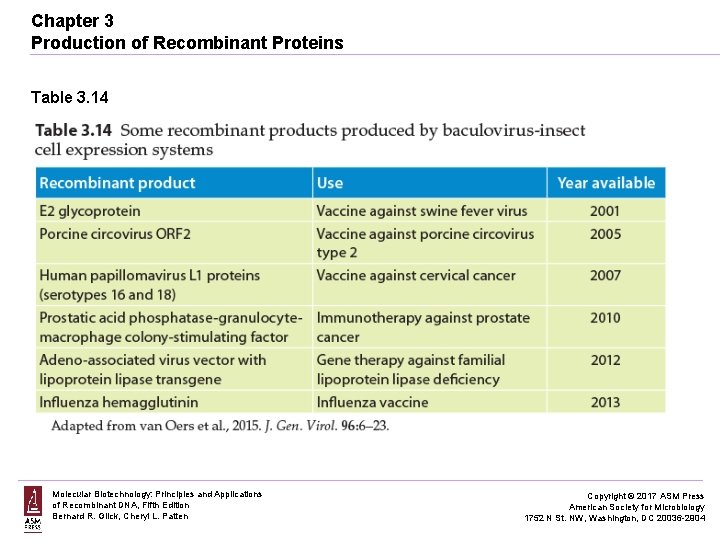
Chapter 3 Production of Recombinant Proteins Table 3. 14 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
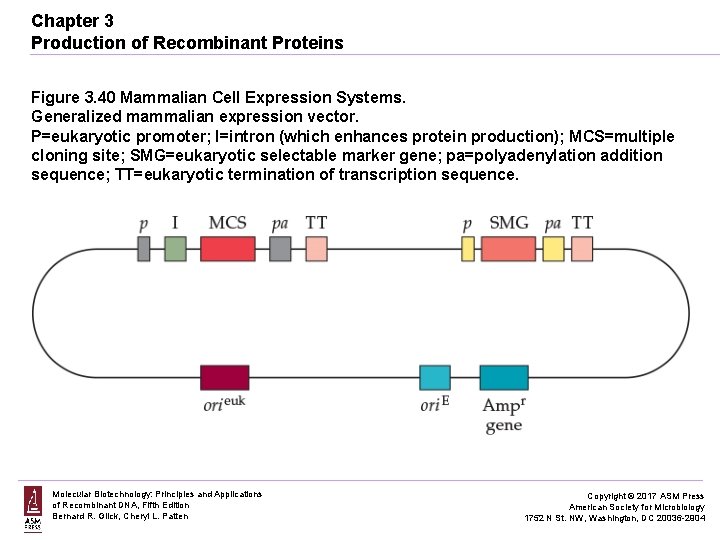
Chapter 3 Production of Recombinant Proteins Figure 3. 40 Mammalian Cell Expression Systems. Generalized mammalian expression vector. P=eukaryotic promoter; I=intron (which enhances protein production); MCS=multiple cloning site; SMG=eukaryotic selectable marker gene; pa=polyadenylation addition sequence; TT=eukaryotic termination of transcription sequence. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
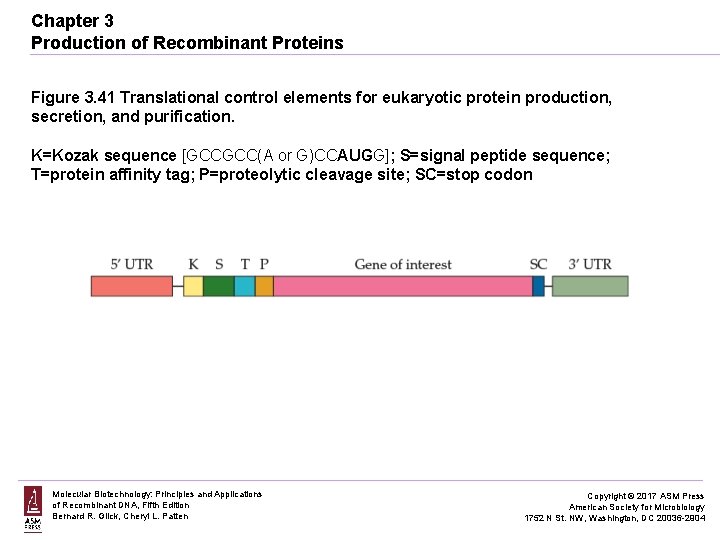
Chapter 3 Production of Recombinant Proteins Figure 3. 41 Translational control elements for eukaryotic protein production, secretion, and purification. K=Kozak sequence [GCCGCC(A or G)CCAUGG]; S=signal peptide sequence; T=protein affinity tag; P=proteolytic cleavage site; SC=stop codon Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
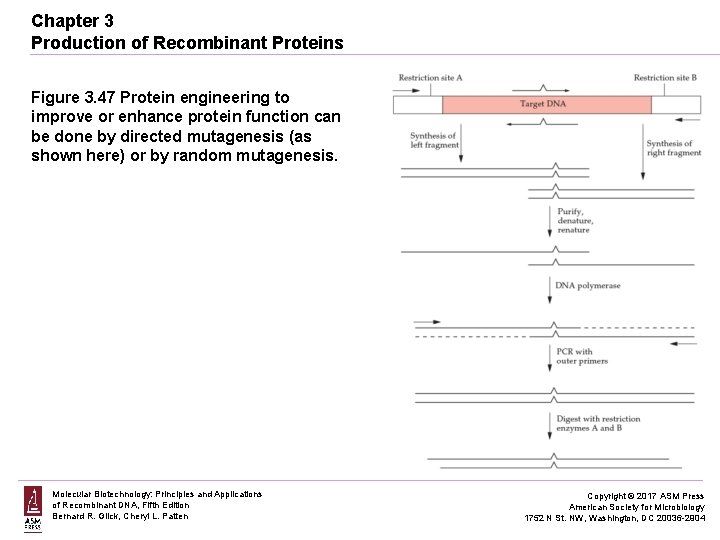
Chapter 3 Production of Recombinant Proteins Figure 3. 47 Protein engineering to improve or enhance protein function can be done by directed mutagenesis (as shown here) or by random mutagenesis. Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
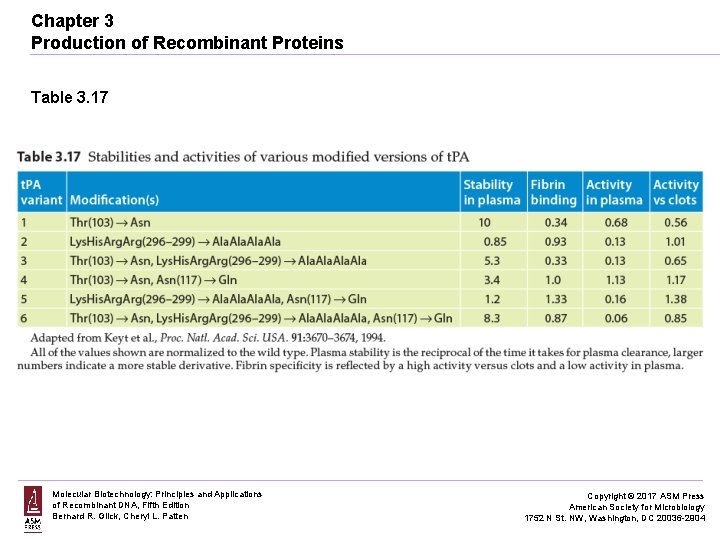
Chapter 3 Production of Recombinant Proteins Table 3. 17 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
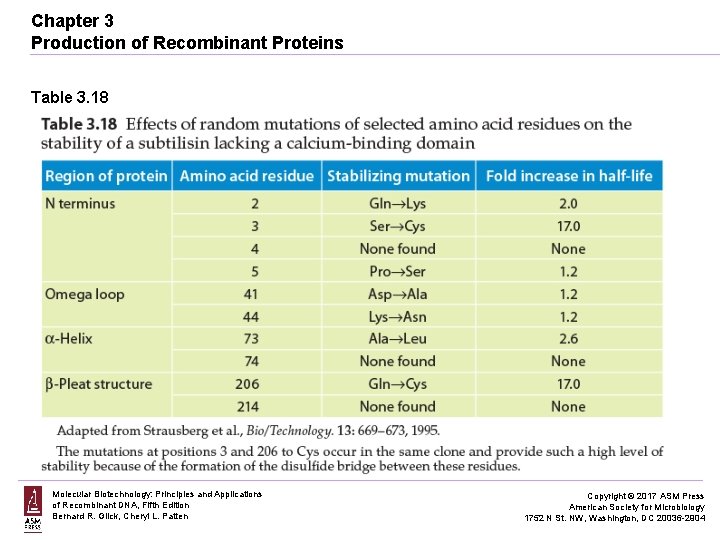
Chapter 3 Production of Recombinant Proteins Table 3. 18 Molecular Biotechnology: Principles and Applications of Recombinant DNA, Fifth Edition Bernard R. Glick, Cheryl L. Patten Copyright © 2017 ASM Press American Society for Microbiology 1752 N St. NW, Washington, DC 20036 -2904
- Slides: 29