Product Design Ferrous and NonFerrous Metals These icons












- Slides: 24

Product Design Ferrous and Non-Ferrous Metals These icons indicate that teacher’s notes or useful web addresses are available in the Notes Page. This icon indicates the slide contains activities created in Flash. These activities are not editable. For more detailed instructions, see the Getting Started presentation. 1 of 24 © Boardworks Ltd 2005

Learning objectives To understand where metals come from and how they are prepared for use. To look at examples of ferrous metals, non-ferrous metals and alloys, and to know the properties of different metals. To understand how heat treatment can change the properties of metals. To be able to use hand tools to work with metals. To be familiar with the industrial processes used to manufacture metal goods. 2 of 24 © Boardworks Ltd 2005

Background on metals Metals are common in manufacturing today. From building and construction work to vehicles and leisure products, they are a vital material for product designers and engineers. The elements of all metals are found naturally in the earth. However, they need to be extracted and processed before they can be used for manufacturing purposes. Because metals in their most basic form are natural resources, designers and manufacturers need to be careful and socially responsible about how much they use, and reuse or recycle metals where possible. 3 of 24 © Boardworks Ltd 2005

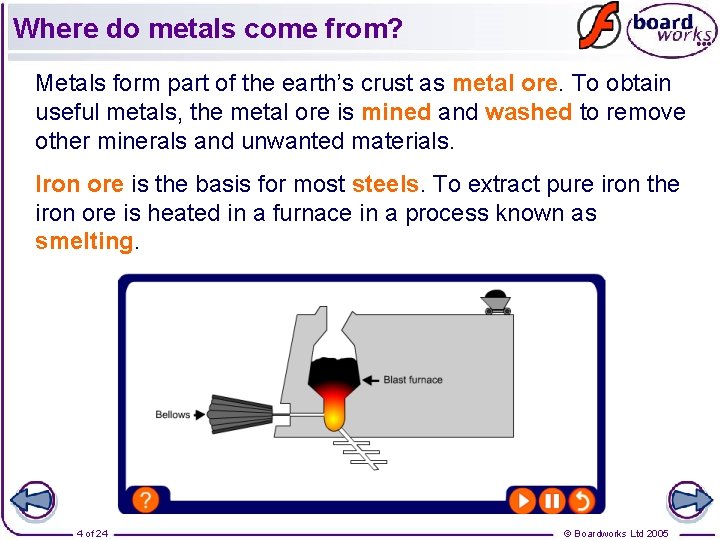
Where do metals come from? Metals form part of the earth’s crust as metal ore. To obtain useful metals, the metal ore is mined and washed to remove other minerals and unwanted materials. Iron ore is the basis for most steels. To extract pure iron the iron ore is heated in a furnace in a process known as smelting. 4 of 24 © Boardworks Ltd 2005

Primary processes – rolling 5 of 24 © Boardworks Ltd 2005

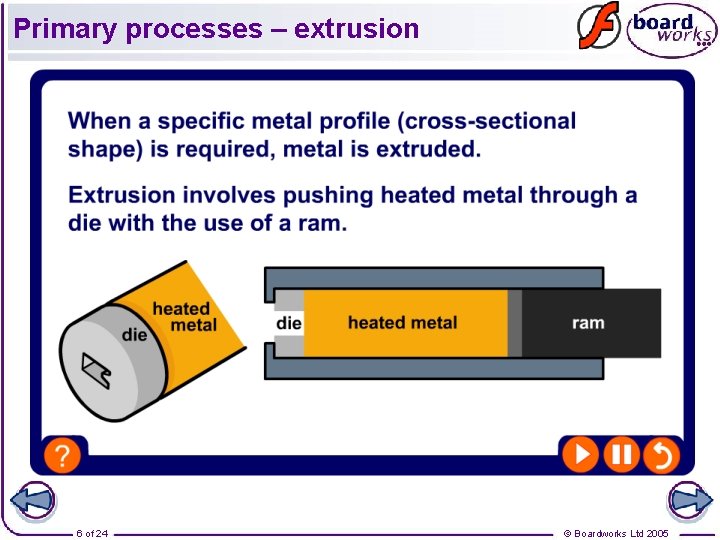
Primary processes – extrusion 6 of 24 © Boardworks Ltd 2005

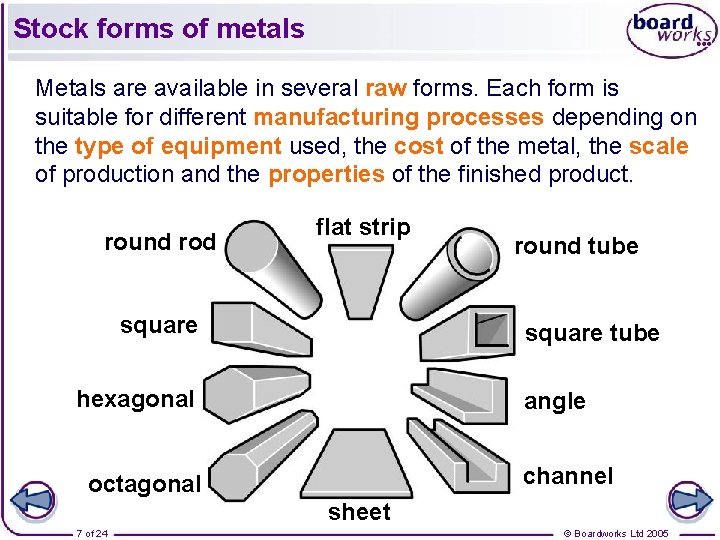
Stock forms of metals Metals are available in several raw forms. Each form is suitable for different manufacturing processes depending on the type of equipment used, the cost of the metal, the scale of production and the properties of the finished product. round rod flat strip square round tube square tube hexagonal angle channel octagonal sheet 7 of 24 © Boardworks Ltd 2005

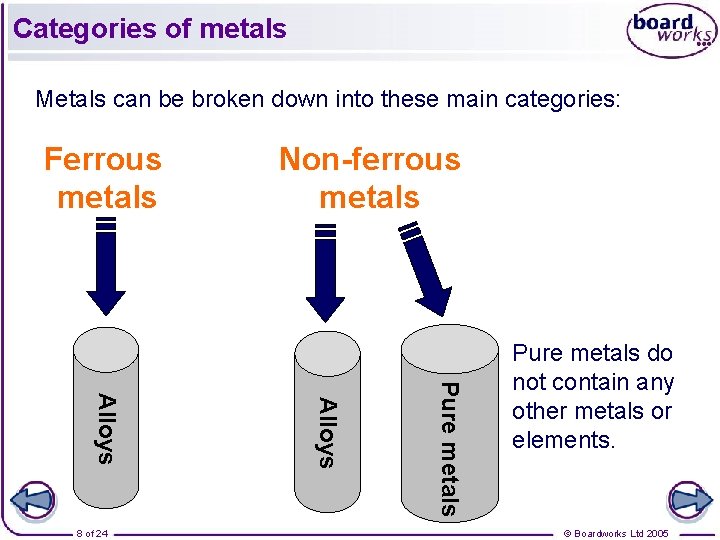
Categories of metals Metals can be broken down into these main categories: Ferrous metals Pure metals Alloys 8 of 24 Non-ferrous metals Pure metals do not contain any other metals or elements. © Boardworks Ltd 2005

Ferrous metals are obtained from iron ore. You might recognize the letters ‘Fe’ from the periodic table, where they represent iron. Ferrous facts Iron replaced bronze as the principal metal by 1000 BC. Early pots and pans made from iron poisoned the users! Early steels were made by adding carbon to iron as it was melted over a charcoal fire. 9 of 24 Ferrous metals: contain iron will corrode unless protected are attracted by a magnet are strong, rigid and cheap. © Boardworks Ltd 2005

A closer look at ferrous metals 10 of 24 © Boardworks Ltd 2005

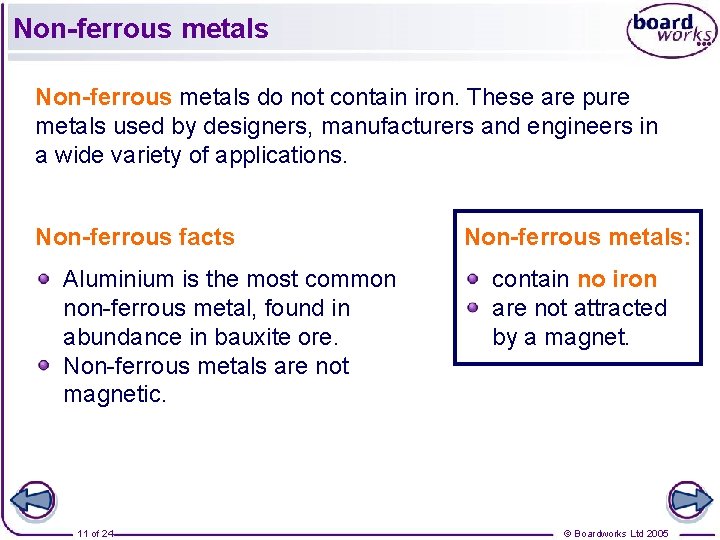
Non-ferrous metals do not contain iron. These are pure metals used by designers, manufacturers and engineers in a wide variety of applications. Non-ferrous facts Aluminium is the most common non-ferrous metal, found in abundance in bauxite ore. Non-ferrous metals are not magnetic. 11 of 24 Non-ferrous metals: contain no iron are not attracted by a magnet. © Boardworks Ltd 2005

A closer look at non-ferrous metals 12 of 24 © Boardworks Ltd 2005

Alloys Sometimes ferrous and non-ferrous metals require different properties in order to function better in specific situations. Alloying metals involves mixing two or more metals and other elements to improve their properties. Alloying metals can: lower the melting point alter thermal and electrical properties make a material harder for cutting purposes improve resistance to corrosion help metal to flow better into a cast. 13 of 24 © Boardworks Ltd 2005

A closer look at alloys 14 of 24 © Boardworks Ltd 2005

Random alloy generator 15 of 24 © Boardworks Ltd 2005

Metals and their properties 16 of 24 © Boardworks Ltd 2005

Changing the properties of metals 17 of 24 © Boardworks Ltd 2005

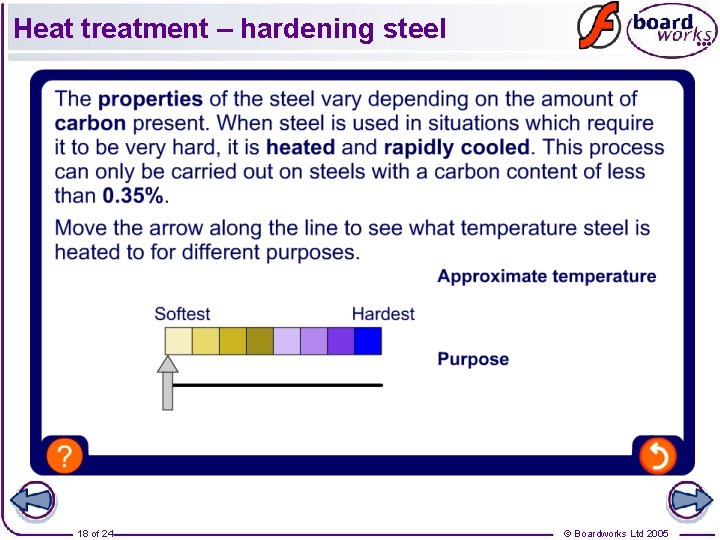
Heat treatment – hardening steel 18 of 24 © Boardworks Ltd 2005

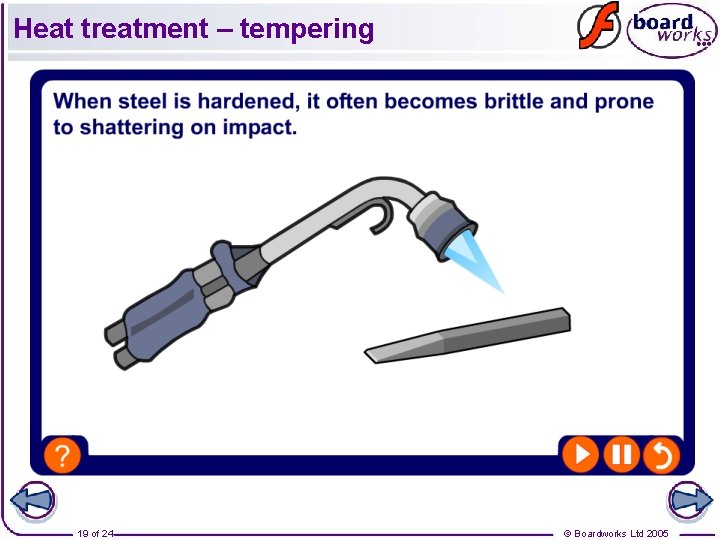
Heat treatment – tempering 19 of 24 © Boardworks Ltd 2005

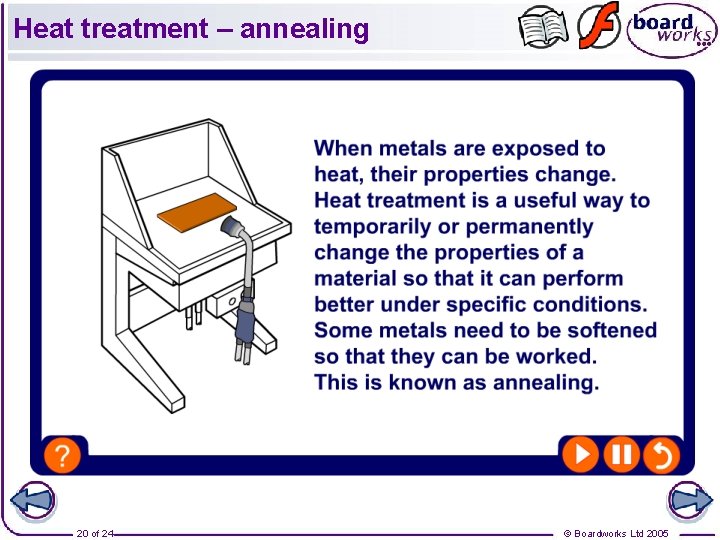
Heat treatment – annealing 20 of 24 © Boardworks Ltd 2005

Working with metals – hand tools 21 of 24 © Boardworks Ltd 2005

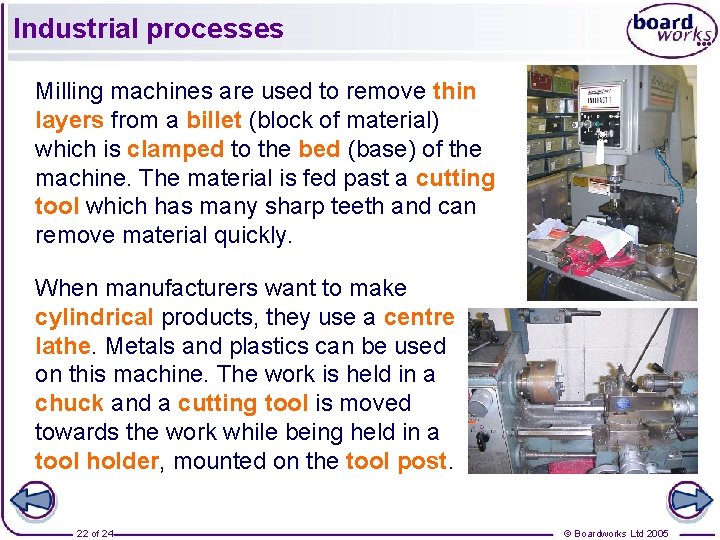
Industrial processes Milling machines are used to remove thin layers from a billet (block of material) which is clamped to the bed (base) of the machine. The material is fed past a cutting tool which has many sharp teeth and can remove material quickly. When manufacturers want to make cylindrical products, they use a centre lathe. Metals and plastics can be used on this machine. The work is held in a chuck and a cutting tool is moved towards the work while being held in a tool holder, mounted on the tool post. 22 of 24 © Boardworks Ltd 2005

Finishing techniques Several surface finishing techniques can be used on metals. The most common ones are detailed below: Paint Surface must be smooth and de-greased Primer required Hammerite is a good one-coat metal paint. Plastic Coating Suitable for most metals Object is heated and dipped in a tank of powder paint Object is returned to oven to ensure a smooth, glossy finish. 23 of 24 Lacquering Helps to prevent corrosion after polishing A layer of cellulose or varnish is applied Often used on jewellery. Enamelling Powdered glass is melted onto the metal surface Provides a hard (but brittle) finish with different colours and textures. © Boardworks Ltd 2005

Key points Metals are extracted from the earth’s crust and then prepared into standard shapes before being sold to manufacturers. Ferrous metals are obtained from iron ore and include cast iron and steel. Non-ferrous metals include aluminium, copper and tin. Alloys, such as brass and stainless steel, are formed from two or more metals and other elements. Different elements alter the properties of metals. Heating metals also alters their properties. Metals can be worked with hand machine tools, including milling machines and centre lathes. 24 of 24 © Boardworks Ltd 2005