Processing of eukaryotic prem RNA For primary transcripts


- Slides: 14

Processing of eukaryotic pre-m. RNA For primary transcripts containing multiple exons and introns, splicing occurs before transcription of the gene is complete--cotranscriptional splicing. Human dystrophin gene has 79 exons, spans over 2, 300 -Kb and requires over 16 hours to be transcribed!

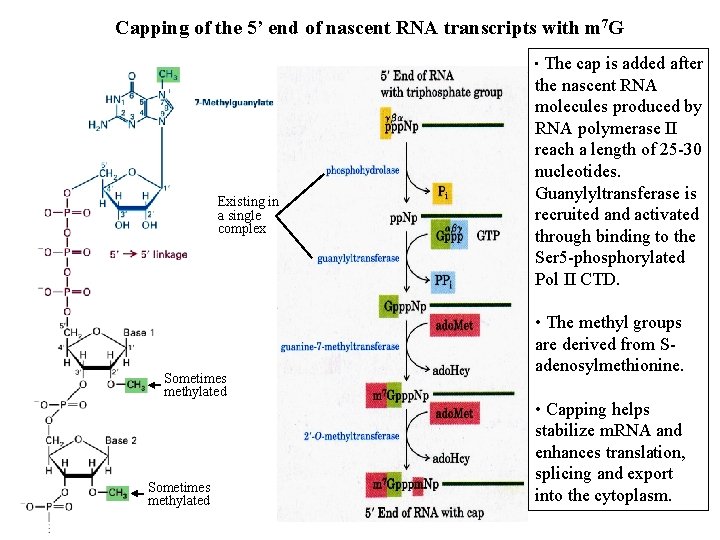
Capping of the 5’ end of nascent RNA transcripts with m 7 G • The Existing in a single complex Sometimes methylated cap is added after the nascent RNA molecules produced by RNA polymerase II reach a length of 25 -30 nucleotides. Guanylyltransferase is recruited and activated through binding to the Ser 5 -phosphorylated Pol II CTD. • The methyl groups are derived from Sadenosylmethionine. • Capping helps stabilize m. RNA and enhances translation, splicing and export into the cytoplasm.

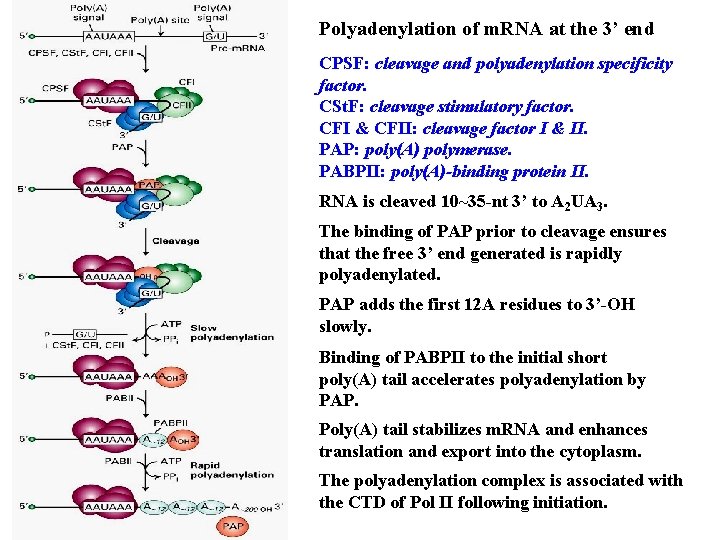
Polyadenylation of m. RNA at the 3’ end CPSF: cleavage and polyadenylation specificity factor. CSt. F: cleavage stimulatory factor. CFI & CFII: cleavage factor I & II. PAP: poly(A) polymerase. PABPII: poly(A)-binding protein II. RNA is cleaved 10~35 -nt 3’ to A 2 UA 3. The binding of PAP prior to cleavage ensures that the free 3’ end generated is rapidly polyadenylated. PAP adds the first 12 A residues to 3’-OH slowly. Binding of PABPII to the initial short poly(A) tail accelerates polyadenylation by PAP. Poly(A) tail stabilizes m. RNA and enhances translation and export into the cytoplasm. The polyadenylation complex is associated with the CTD of Pol II following initiation.

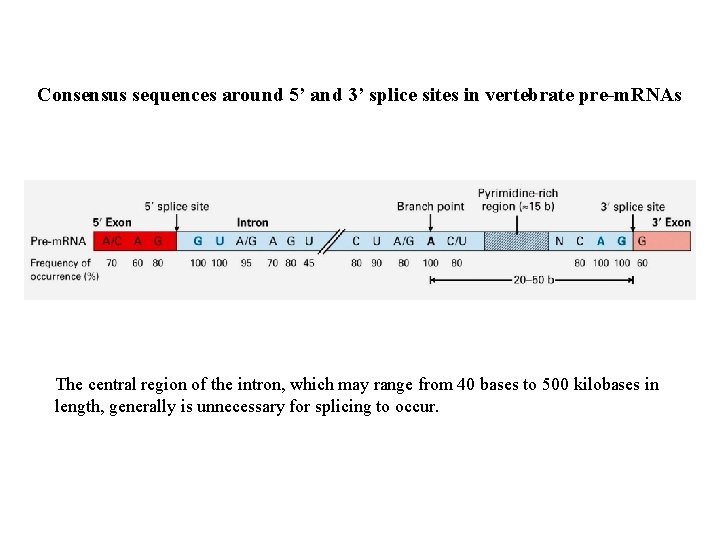
Consensus sequences around 5’ and 3’ splice sites in vertebrate pre-m. RNAs The central region of the intron, which may range from 40 bases to 500 kilobases in length, generally is unnecessary for splicing to occur.

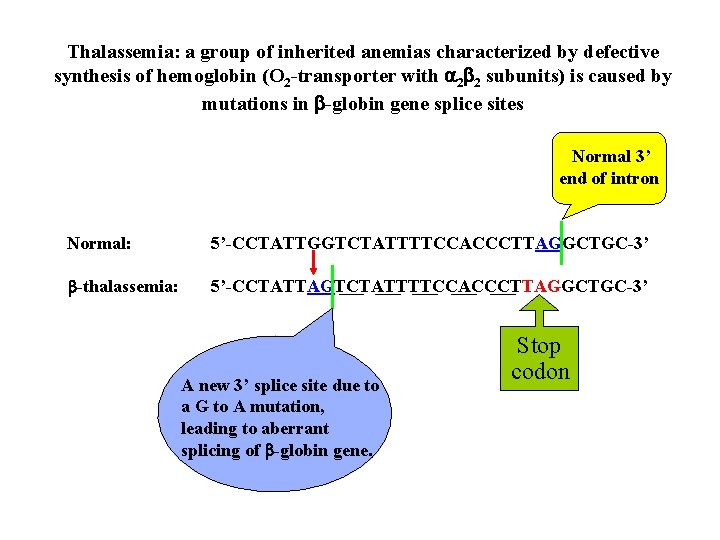
Thalassemia: a group of inherited anemias characterized by defective synthesis of hemoglobin (O 2 -transporter with 2 2 subunits) is caused by mutations in -globin gene splice sites Normal 3’ end of intron Normal: 5’-CCTATTGGTCTATTTTCCACCCTTAGGCTGC-3’ -thalassemia: 5’-CCTATTAGTCTATTTTCCACCCTTAGGCTGC-3’ A new 3’ splice site due to a G to A mutation, leading to aberrant splicing of -globin gene. Stop codon

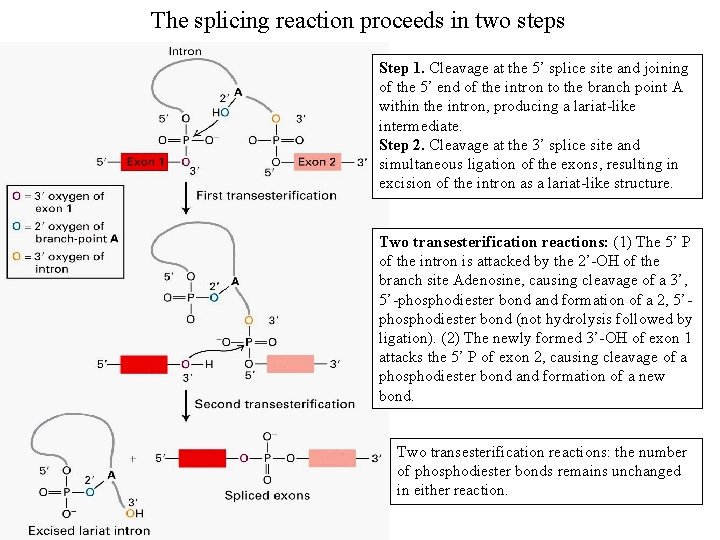
The splicing reaction proceeds in two steps Step 1. Cleavage at the 5’ splice site and joining of the 5’ end of the intron to the branch point A within the intron, producing a lariat-like intermediate. Step 2. Cleavage at the 3’ splice site and simultaneous ligation of the exons, resulting in excision of the intron as a lariat-like structure. Two transesterification reactions: (1) The 5’ P of the intron is attacked by the 2’-OH of the branch site Adenosine, causing cleavage of a 3’, 5’-phosphodiester bond and formation of a 2, 5’phosphodiester bond (not hydrolysis followed by ligation). (2) The newly formed 3’-OH of exon 1 attacks the 5’ P of exon 2, causing cleavage of a phosphodiester bond and formation of a new bond. Two transesterification reactions: the number of phosphodiester bonds remains unchanged in either reaction.

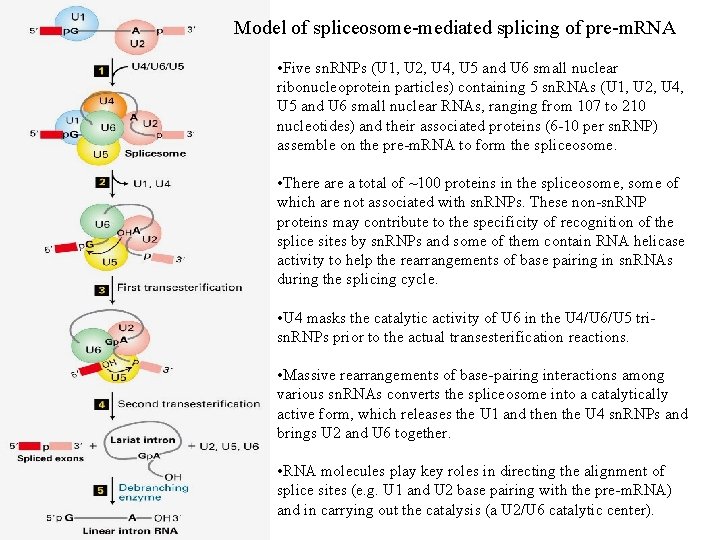
Model of spliceosome-mediated splicing of pre-m. RNA • Five sn. RNPs (U 1, U 2, U 4, U 5 and U 6 small nuclear ribonucleoprotein particles) containing 5 sn. RNAs (U 1, U 2, U 4, U 5 and U 6 small nuclear RNAs, ranging from 107 to 210 nucleotides) and their associated proteins (6 -10 per sn. RNP) assemble on the pre-m. RNA to form the spliceosome. • There a total of ~100 proteins in the spliceosome, some of which are not associated with sn. RNPs. These non-sn. RNP proteins may contribute to the specificity of recognition of the splice sites by sn. RNPs and some of them contain RNA helicase activity to help the rearrangements of base pairing in sn. RNAs during the splicing cycle. • U 4 masks the catalytic activity of U 6 in the U 4/U 6/U 5 trisn. RNPs prior to the actual transesterification reactions. • Massive rearrangements of base-pairing interactions among various sn. RNAs converts the spliceosome into a catalytically active form, which releases the U 1 and then the U 4 sn. RNPs and brings U 2 and U 6 together. • RNA molecules play key roles in directing the alignment of splice sites (e. g. U 1 and U 2 base pairing with the pre-m. RNA) and in carrying out the catalysis (a U 2/U 6 catalytic center).

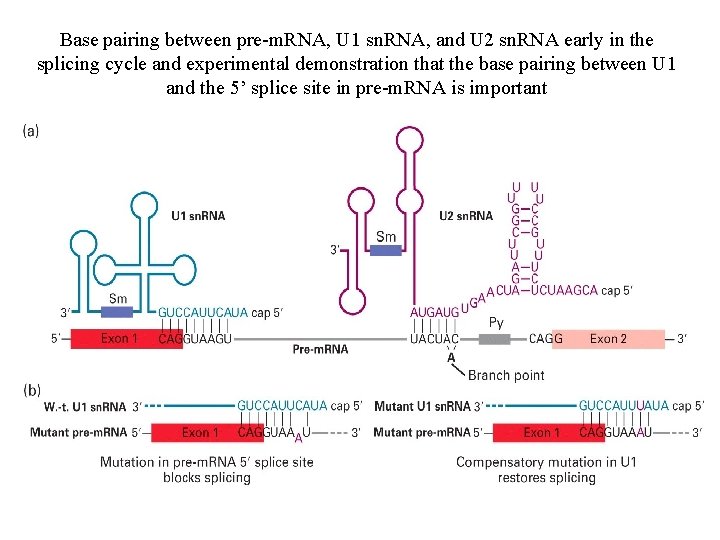
Base pairing between pre-m. RNA, U 1 sn. RNA, and U 2 sn. RNA early in the splicing cycle and experimental demonstration that the base pairing between U 1 and the 5’ splice site in pre-m. RNA is important

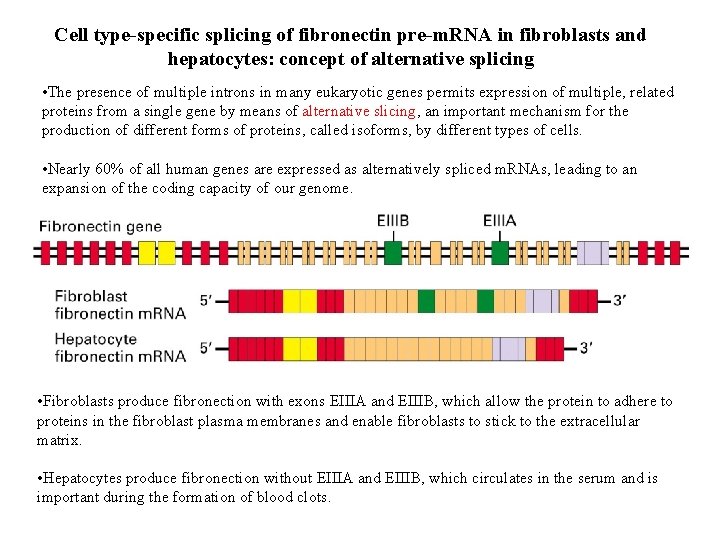
Cell type-specific splicing of fibronectin pre-m. RNA in fibroblasts and hepatocytes: concept of alternative splicing • The presence of multiple introns in many eukaryotic genes permits expression of multiple, related proteins from a single gene by means of alternative slicing, an important mechanism for the production of different forms of proteins, called isoforms, by different types of cells. • Nearly 60% of all human genes are expressed as alternatively spliced m. RNAs, leading to an expansion of the coding capacity of our genome. • Fibroblasts produce fibronection with exons EIIIA and EIIIB, which allow the protein to adhere to proteins in the fibroblast plasma membranes and enable fibroblasts to stick to the extracellular matrix. • Hepatocytes produce fibronection without EIIIA and EIIIB, which circulates in the serum and is important during the formation of blood clots.

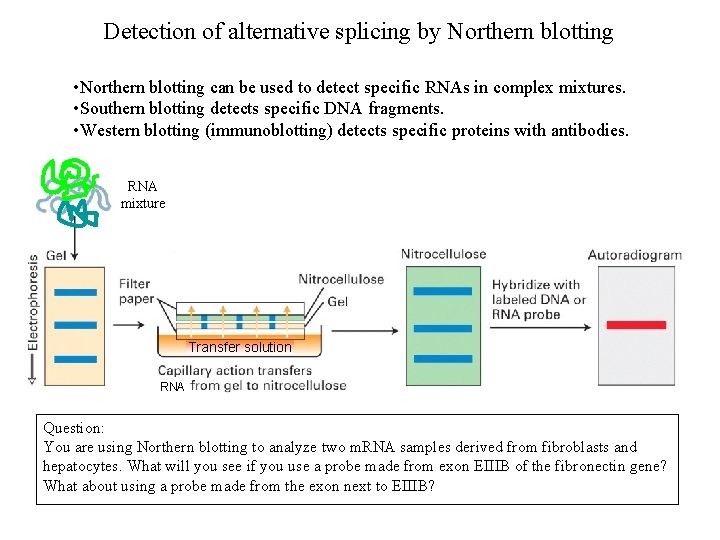
Detection of alternative splicing by Northern blotting • Northern blotting can be used to detect specific RNAs in complex mixtures. • Southern blotting detects specific DNA fragments. • Western blotting (immunoblotting) detects specific proteins with antibodies. RNA mixture Transfer solution RNA Question: You are using Northern blotting to analyze two m. RNA samples derived from fibroblasts and hepatocytes. What will you see if you use a probe made from exon EIIIB of the fibronectin gene? What about using a probe made from the exon next to EIIIB?

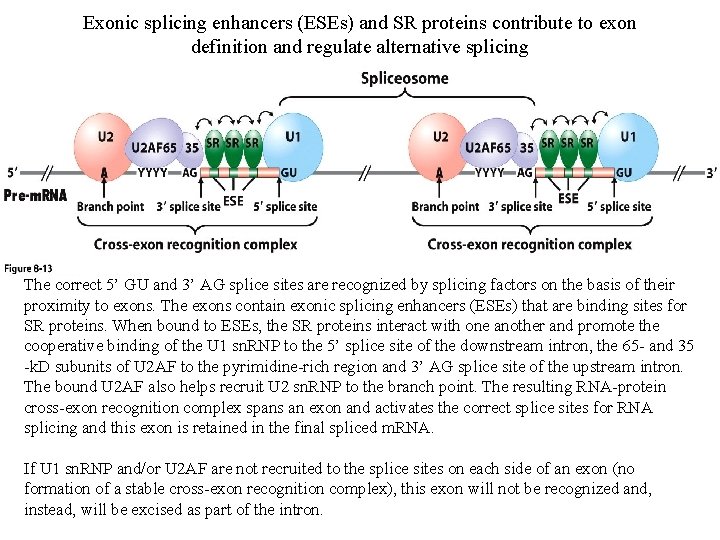
Exonic splicing enhancers (ESEs) and SR proteins contribute to exon definition and regulate alternative splicing The correct 5’ GU and 3’ AG splice sites are recognized by splicing factors on the basis of their proximity to exons. The exons contain exonic splicing enhancers (ESEs) that are binding sites for SR proteins. When bound to ESEs, the SR proteins interact with one another and promote the cooperative binding of the U 1 sn. RNP to the 5’ splice site of the downstream intron, the 65 - and 35 -k. D subunits of U 2 AF to the pyrimidine-rich region and 3’ AG splice site of the upstream intron. The bound U 2 AF also helps recruit U 2 sn. RNP to the branch point. The resulting RNA-protein cross-exon recognition complex spans an exon and activates the correct splice sites for RNA splicing and this exon is retained in the final spliced m. RNA. If U 1 sn. RNP and/or U 2 AF are not recruited to the splice sites on each side of an exon (no formation of a stable cross-exon recognition complex), this exon will not be recognized and, instead, will be excised as part of the intron.

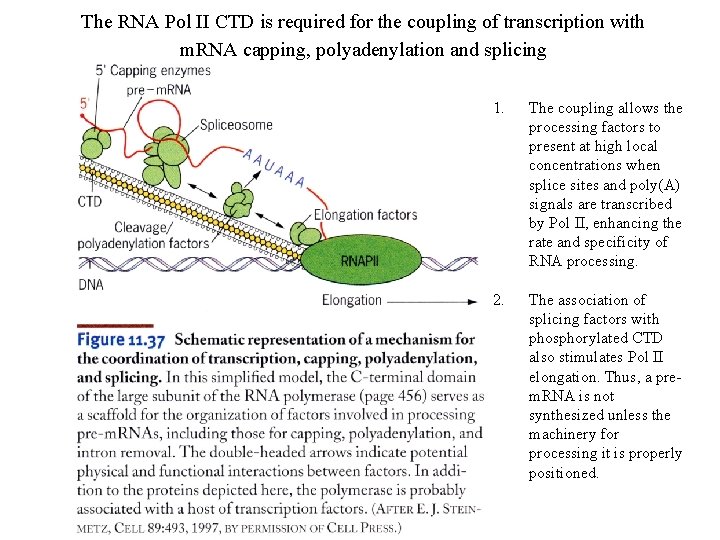
The RNA Pol II CTD is required for the coupling of transcription with m. RNA capping, polyadenylation and splicing 1. The coupling allows the processing factors to present at high local concentrations when splice sites and poly(A) signals are transcribed by Pol II, enhancing the rate and specificity of RNA processing. 2. The association of splicing factors with phosphorylated CTD also stimulates Pol II elongation. Thus, a prem. RNA is not synthesized unless the machinery for processing it is properly positioned.

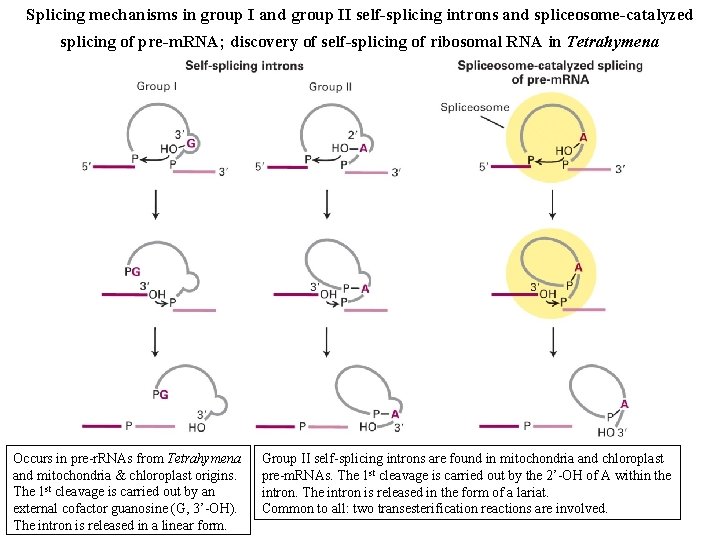
Splicing mechanisms in group I and group II self-splicing introns and spliceosome-catalyzed splicing of pre-m. RNA; discovery of self-splicing of ribosomal RNA in Tetrahymena Occurs in pre-r. RNAs from Tetrahymena and mitochondria & chloroplast origins. The 1 st cleavage is carried out by an external cofactor guanosine (G, 3’-OH). The intron is released in a linear form. Group II self-splicing introns are found in mitochondria and chloroplast pre-m. RNAs. The 1 st cleavage is carried out by the 2’-OH of A within the intron. The intron is released in the form of a lariat. Common to all: two transesterification reactions are involved.

Catalytic introns ----- RNA as enzymes ------ Ribozymes During evolution, there has been a transfer of catalytic power from the intron itself to other molecules such as sn. RNPs, which are specialized in carrying out splicing reactions. Introns nowadays are variable in size because they are no longer selfsplicing and this increases the capacity of regulation. The similarity between these two suggests that the U sn. RNAs probably evolved from group II introns of endosymbiotic organelles.