Processes of Science Scientific Method 1 Observation of

Processes of Science Scientific Method 1. Observation of natural phenomena 2. Researching information 3. Formulating a hypothesis 4. Designing and performing an experiment 5. Analyzing data to either support or reject the hypothesis in step 3 6. Reporting the results 2 -1

How do we obtain evidence that can support our hypothesis? 2 -2

Chapter 2: The Molecules of Cells 2 -3
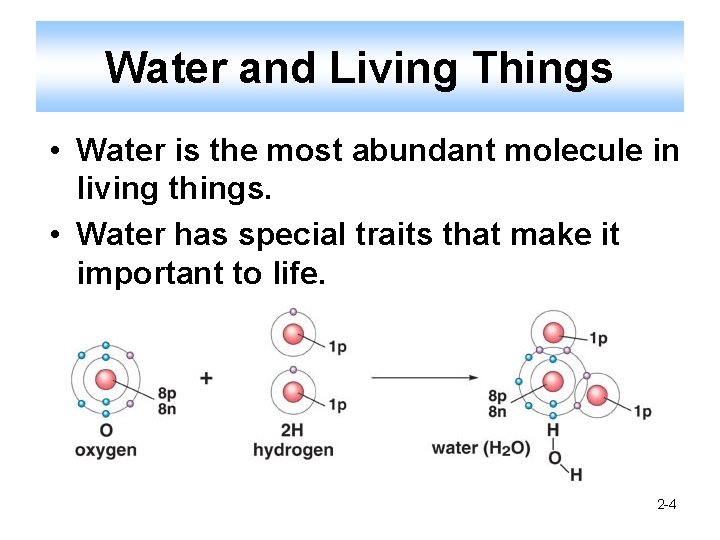
Water and Living Things • Water is the most abundant molecule in living things. • Water has special traits that make it important to life. 2 -4
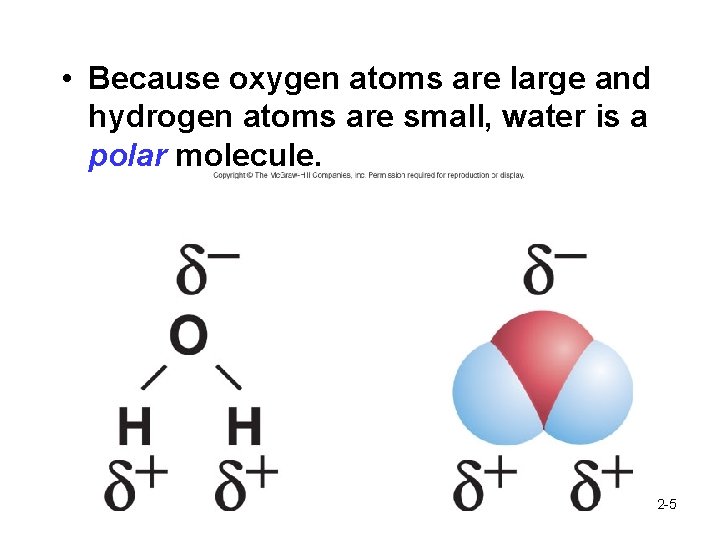
• Because oxygen atoms are large and hydrogen atoms are small, water is a polar molecule. 2 -5

• Hydrogen bonds form when a covalently-bonded H+ is attracted to a negatively-charged atom in a neighboring molecule. • Because of its polarity and hydrogen bonding, water has unique characteristics that benefit living things. 2 -6

Characteristics of water: 1. 2. 3. 4. 5. 6. liquid at room temperature universal solvent for polar molecules water molecules are cohesive temperature of water changes slowly high heat of vaporization frozen water is less dense so ice floats 2 -7
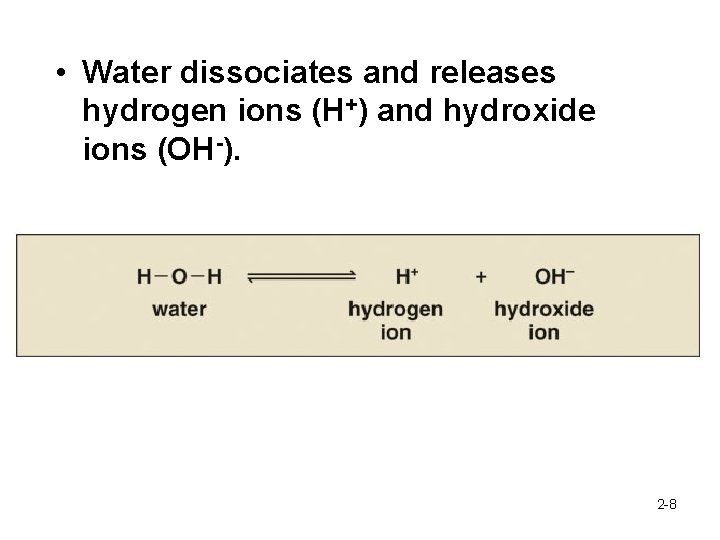
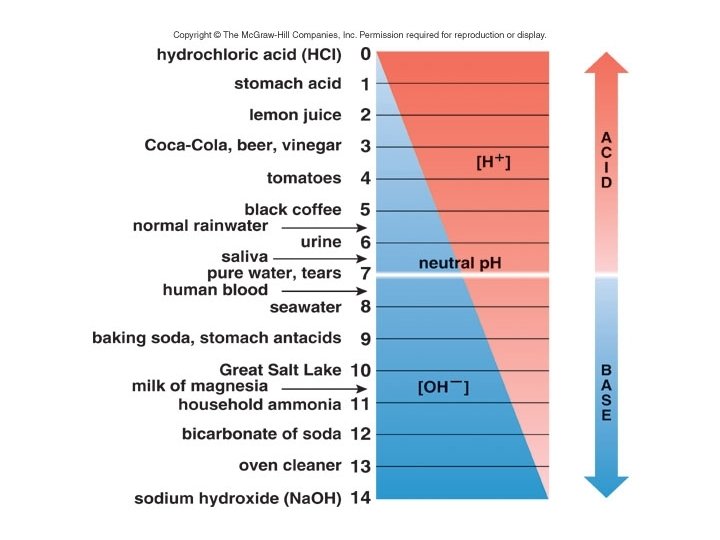
• Water dissociates and releases hydrogen ions (H+) and hydroxide ions (OH-). 2 -8

• Acids are molecules that release hydrogen ions in solution. HCl H+ + Cl- 2 -9
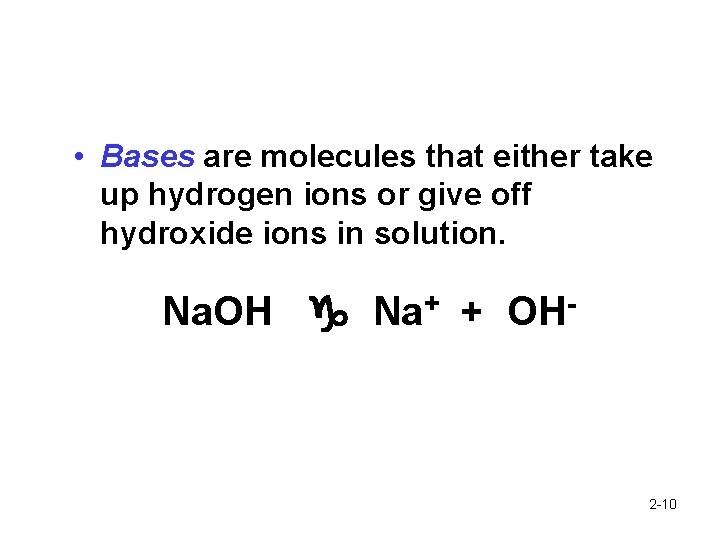
• Bases are molecules that either take up hydrogen ions or give off hydroxide ions in solution. Na. OH Na+ + OH- 2 -10
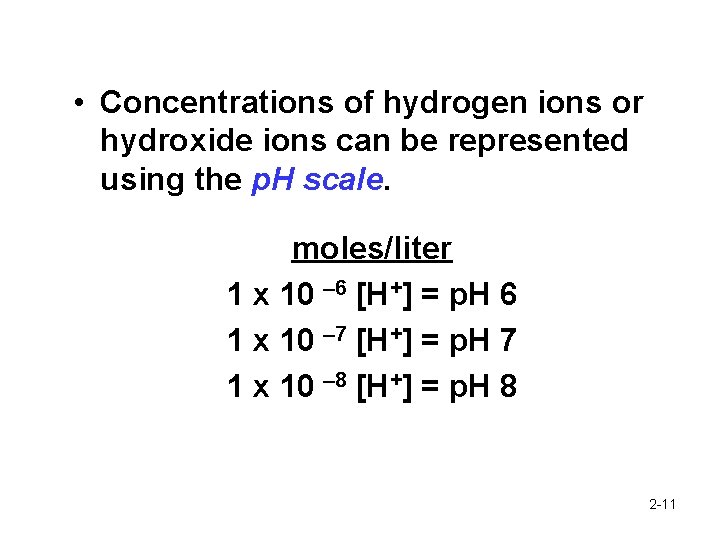
• Concentrations of hydrogen ions or hydroxide ions can be represented using the p. H scale. moles/liter 1 x 10 – 6 [H+] = p. H 6 1 x 10 – 7 [H+] = p. H 7 1 x 10 – 8 [H+] = p. H 8 2 -11
2 -12
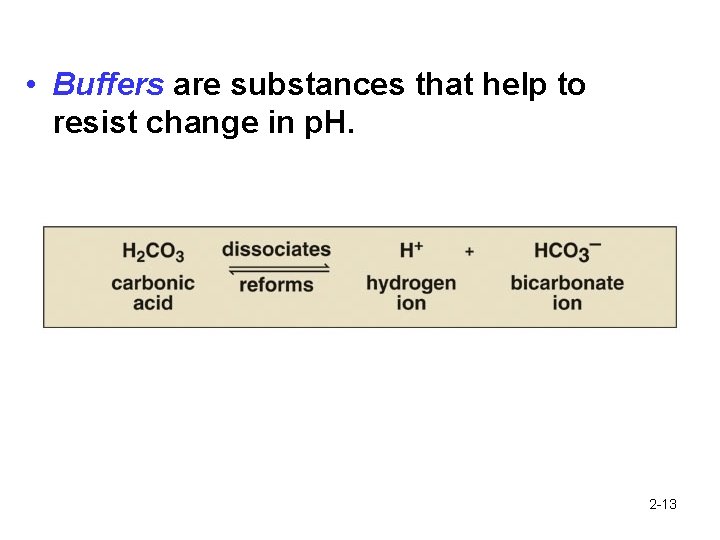
• Buffers are substances that help to resist change in p. H. 2 -13

Discussion: What is the role of bicarbonate ions in the blood? Explain the effect of adding HCl to a system buffered with HCO 31 - 2 -14
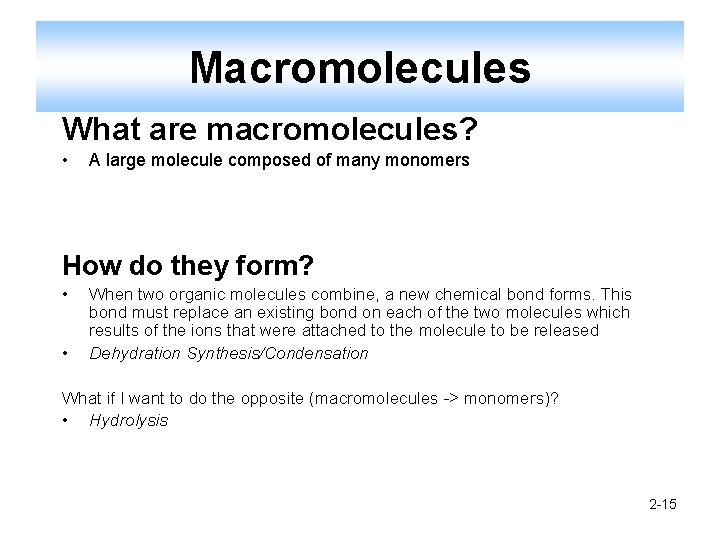
Macromolecules What are macromolecules? • A large molecule composed of many monomers How do they form? • • When two organic molecules combine, a new chemical bond forms. This bond must replace an existing bond on each of the two molecules which results of the ions that were attached to the molecule to be released Dehydration Synthesis/Condensation What if I want to do the opposite (macromolecules -> monomers)? • Hydrolysis 2 -15
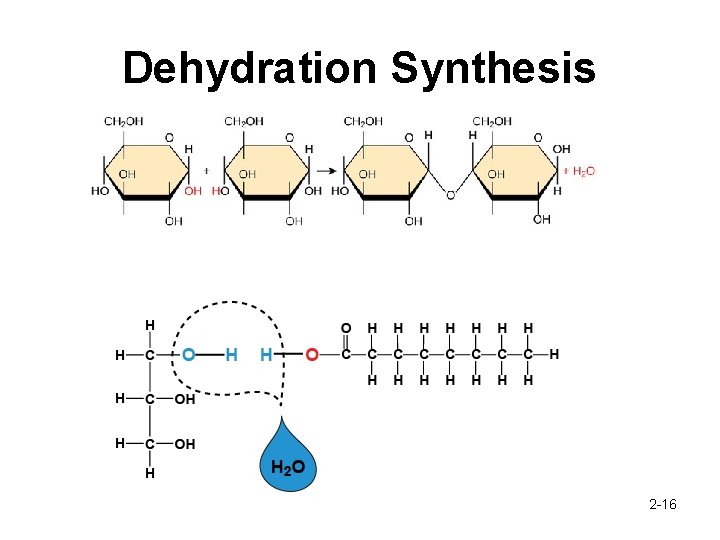
Dehydration Synthesis 2 -16
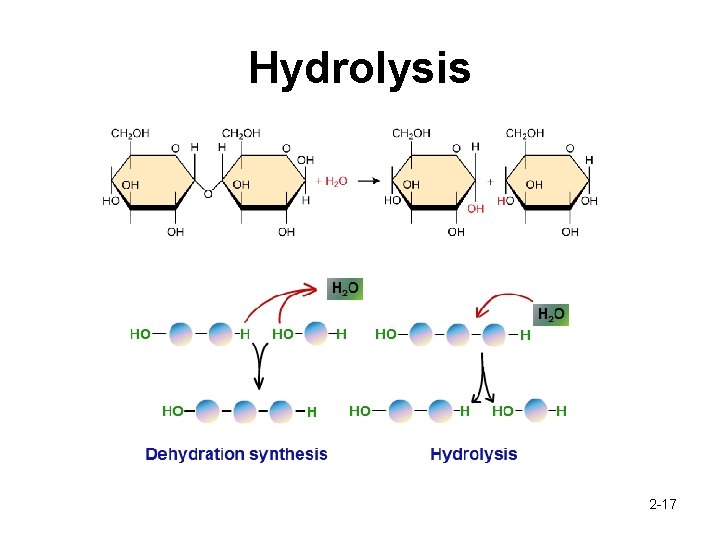
Hydrolysis 2 -17

Macromolecules (polymers) are formed from smaller building blocks called monomers. Polymer carbohydrate protein lipid nucleic acid Monomer monosaccharides amino acid glycerol & fatty acids nucleotide 2 -18
Carbohydrates serve as quick energy and short-term energy storage. They play a structural role in plants, bacteria, and insects. Monomers of carbohydrates are the monosaccharides: glucose fructose galactose 2 -19
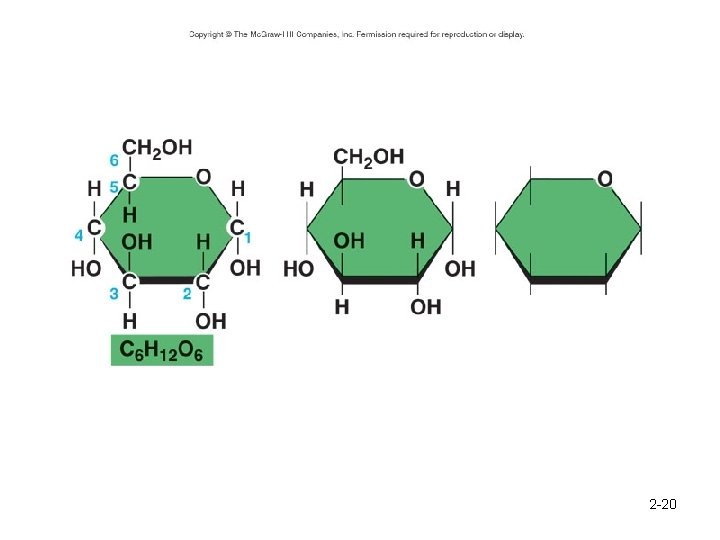
Structure of Glucose 2 -20
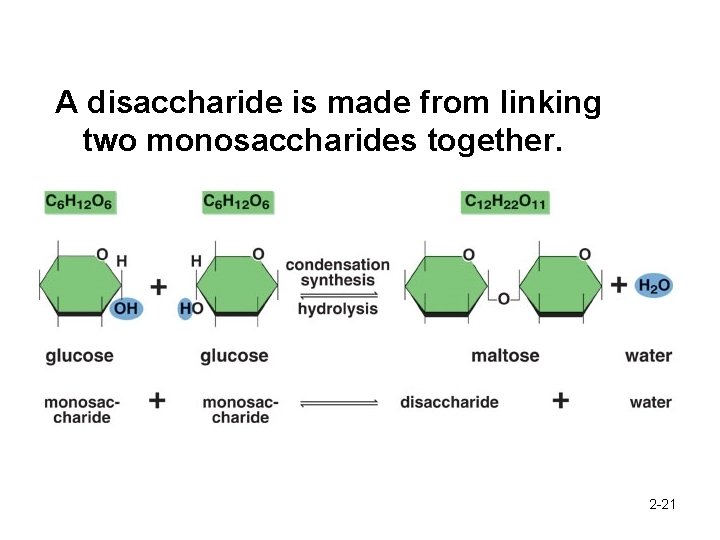
A disaccharide is made from linking two monosaccharides together. 2 -21

What is a disaccharide composed of? • Di = 2 • 2 or more saccharides joined together • Maltose (glucose-glucose) • Sucrose (glucose-fructose) What is a polysaccharide? • Poly = 3 or more glucose joined together • Also called complex carbohydrate 2 -22

Larger polysaccharides are made from linking many glucose molecules together through dehydration synthesis or condensation synthesis. Examples of polysaccharides: Starch glycogen cellulose 2 -23
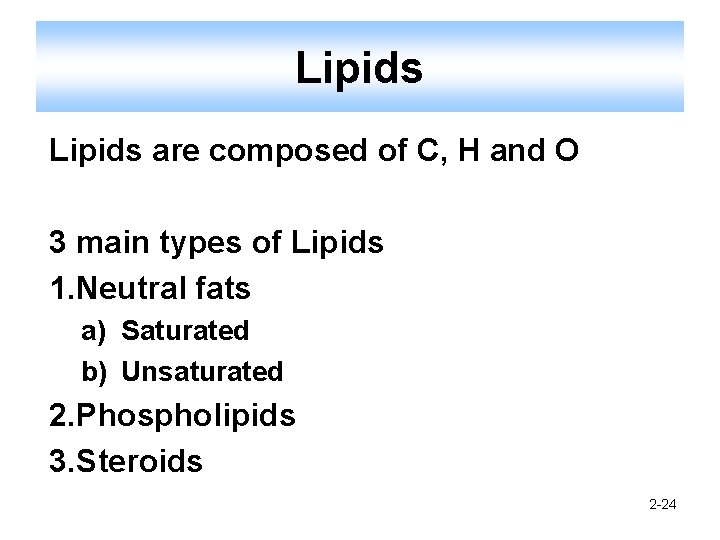
Lipids are composed of C, H and O 3 main types of Lipids 1. Neutral fats a) Saturated b) Unsaturated 2. Phospholipids 3. Steroids 2 -24

1. Neutral Fats • Not a polymer • Also called triglycerides • Function – Long term energy store insulation – Covers and protects internal organs (ie: kidneys and eyes) – Composed of 1 glycerol + 3 FA 2 -25
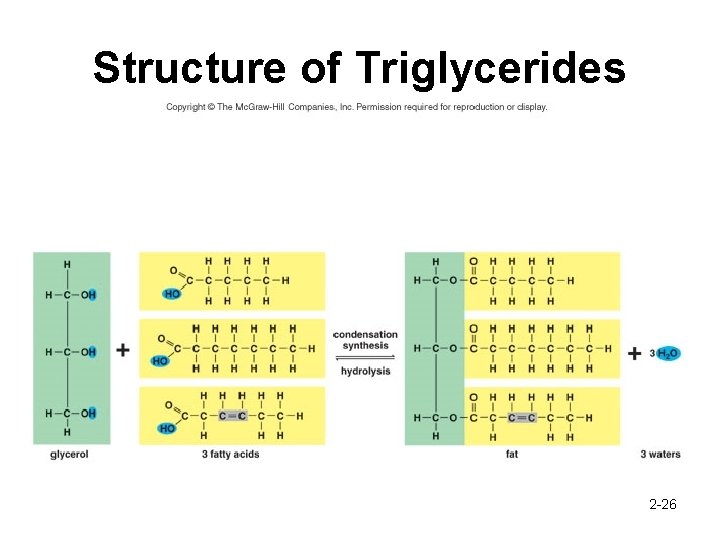
Structure of Triglycerides 2 -26
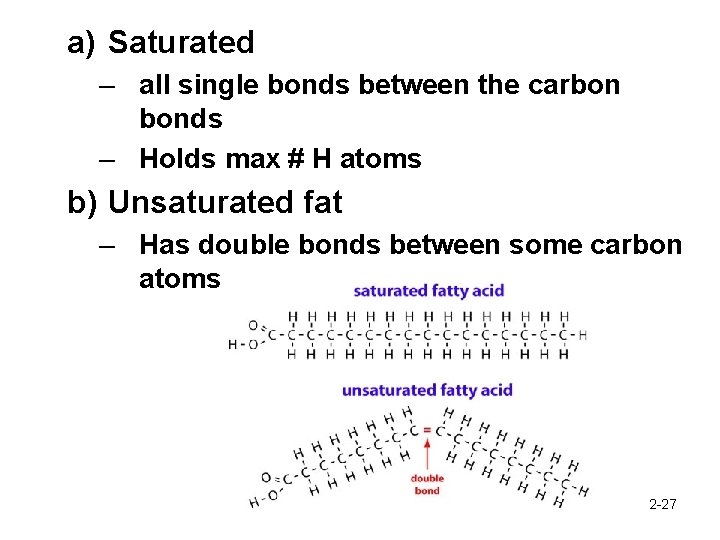
a) Saturated – all single bonds between the carbon bonds – Holds max # H atoms b) Unsaturated fat – Has double bonds between some carbon atoms 2 -27
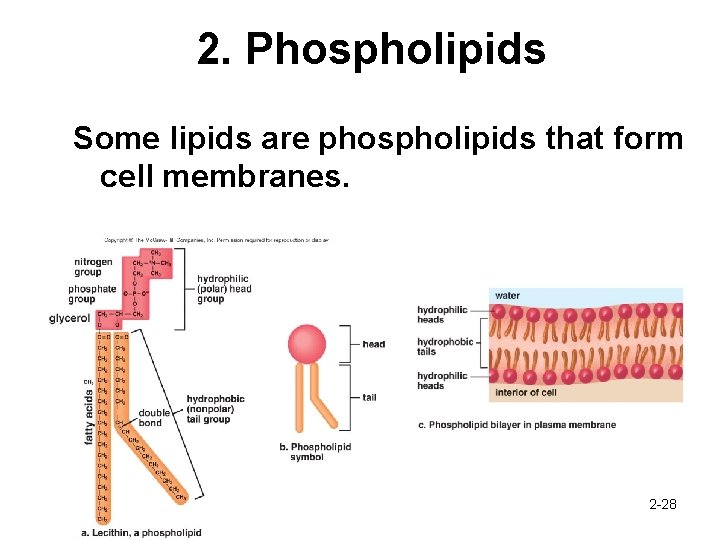
2. Phospholipids Some lipids are phospholipids that form cell membranes. 2 -28
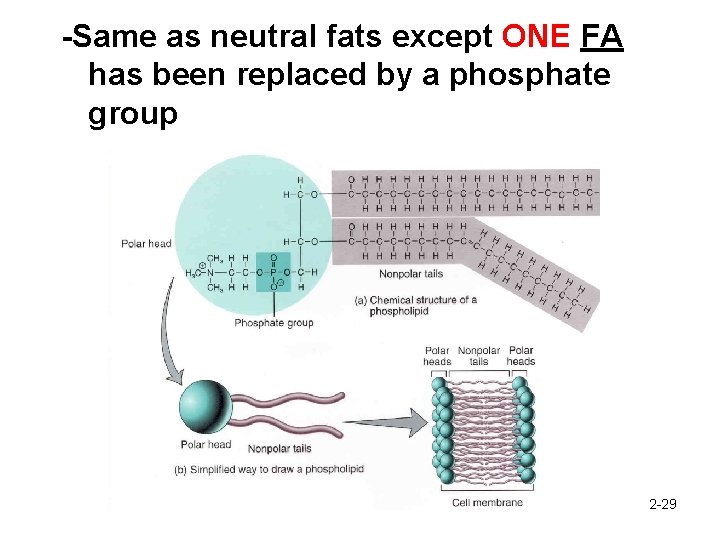
-Same as neutral fats except ONE FA has been replaced by a phosphate group 2 -29

3. Steroids -made up of 4 fused rings of carbon atoms + R group Examples include cholesterol, and the sex hormones estrogen and testosterone. 2 -30
Proteins perform many functions in cells. Proteins: -Serve as structural proteins -Act as enzymes to speed reactions -Serve as transport carriers -Act as antibodies -Allow materials to cross cell membranes 2 -31
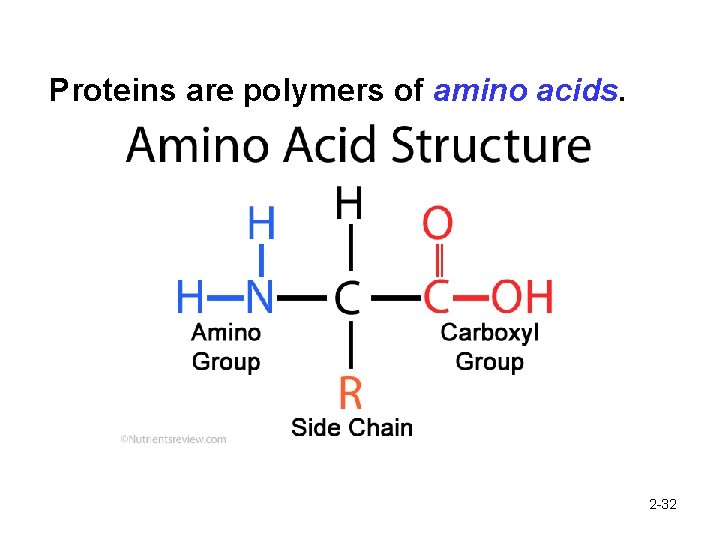
Proteins are polymers of amino acids. 2 -32
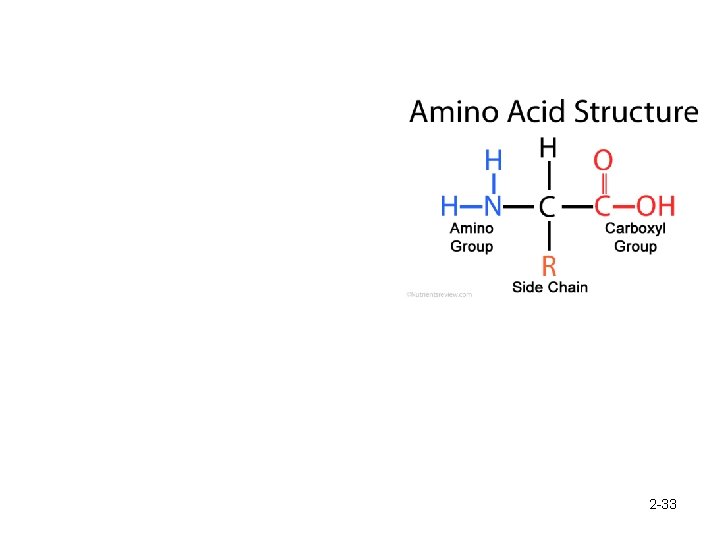
2 -33
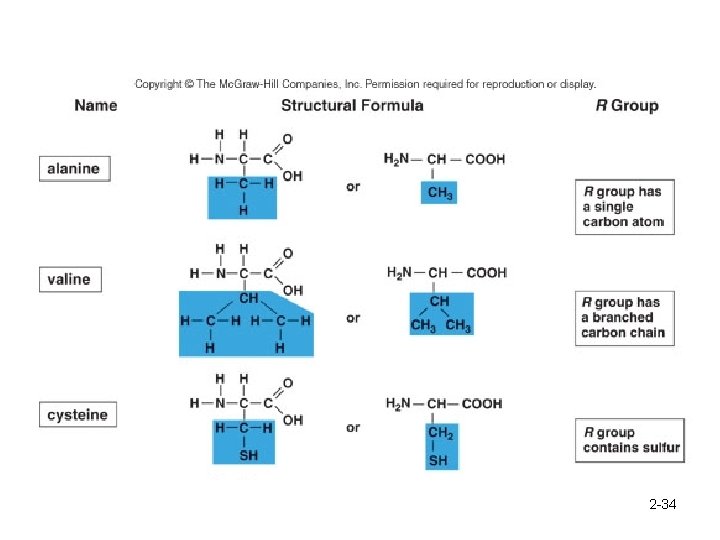
2 -34
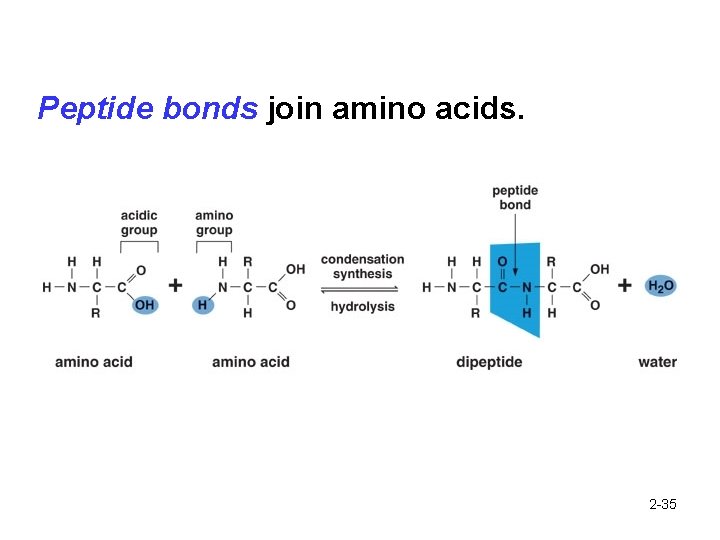
Peptide bonds join amino acids. 2 -35
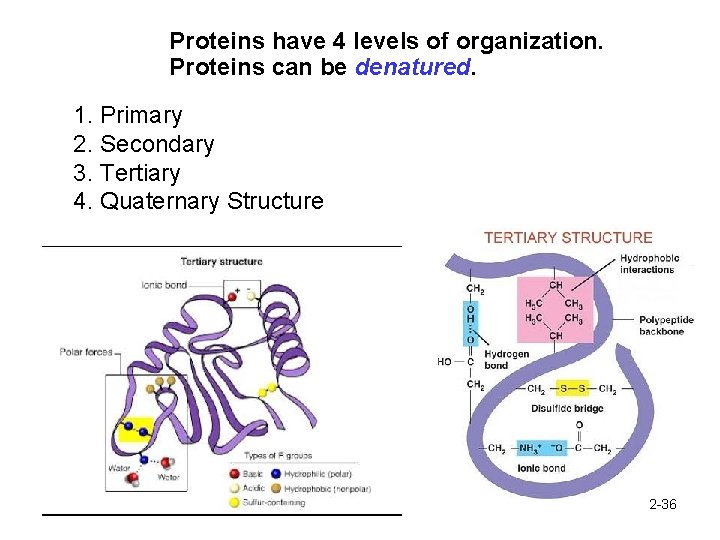
Proteins have 4 levels of organization. Proteins can be denatured. 1. Primary 2. Secondary 3. Tertiary 4. Quaternary Structure 2 -36
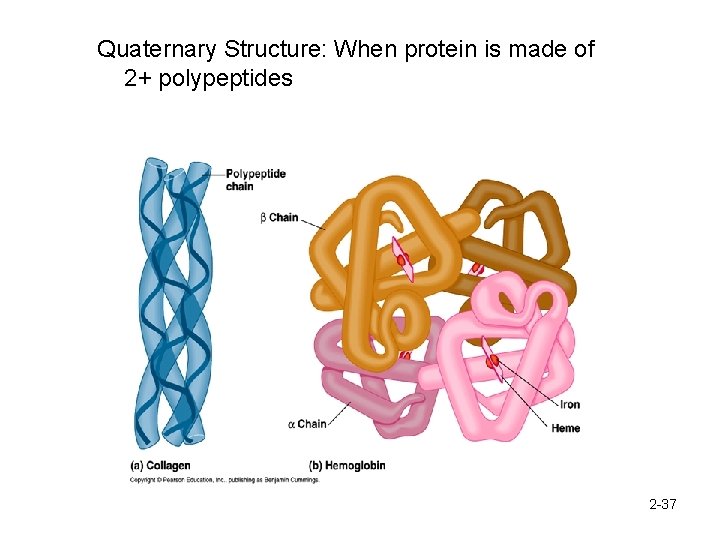
Quaternary Structure: When protein is made of 2+ polypeptides 2 -37
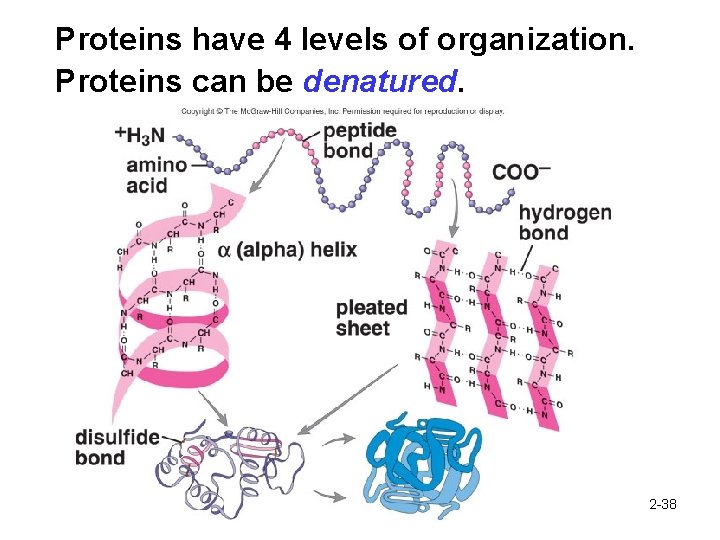
Proteins have 4 levels of organization. Proteins can be denatured. 2 -38
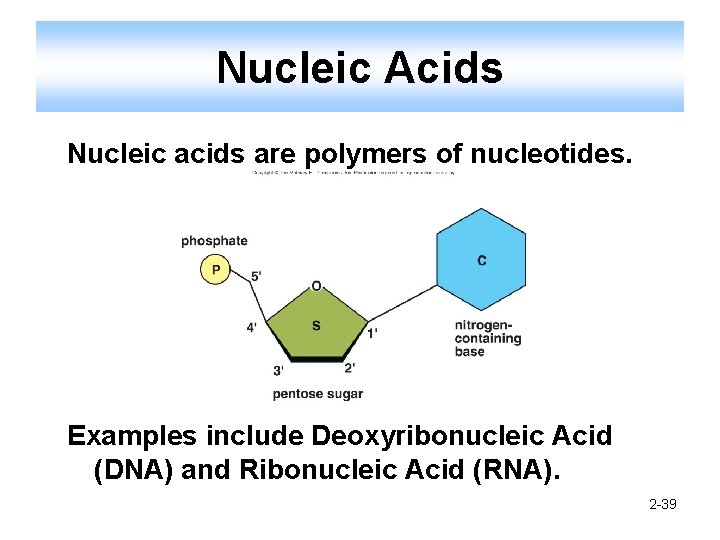
Nucleic Acids Nucleic acids are polymers of nucleotides. Examples include Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA). 2 -39
2 -40
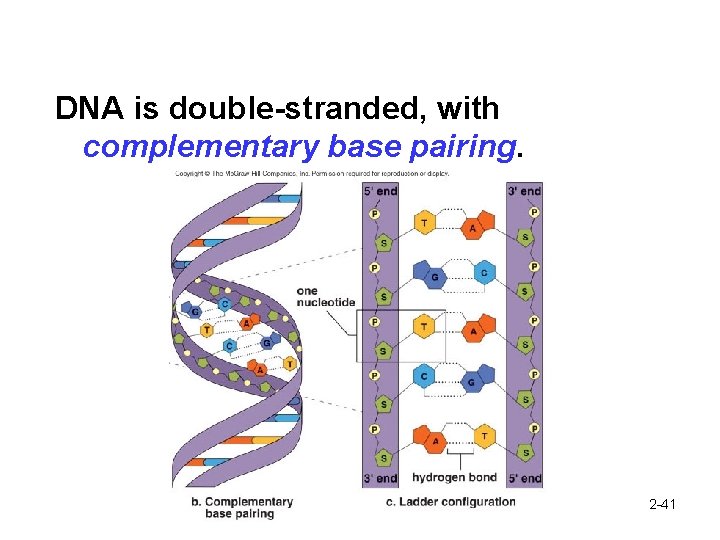
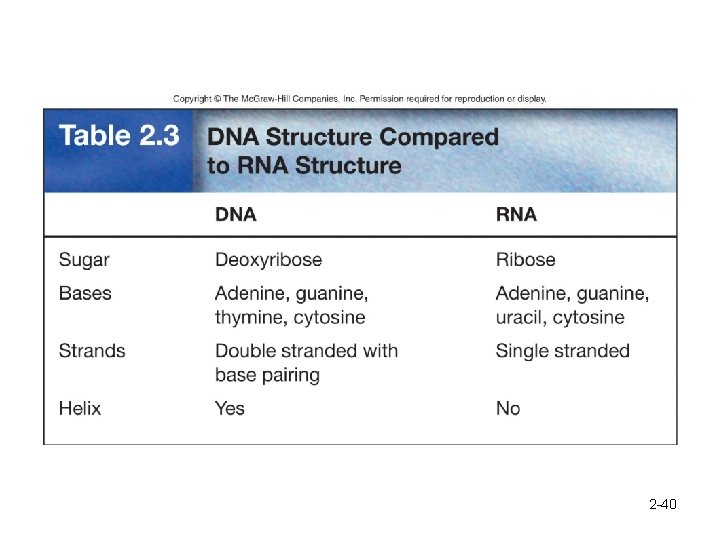
DNA is double-stranded, with complementary base pairing. 2 -41
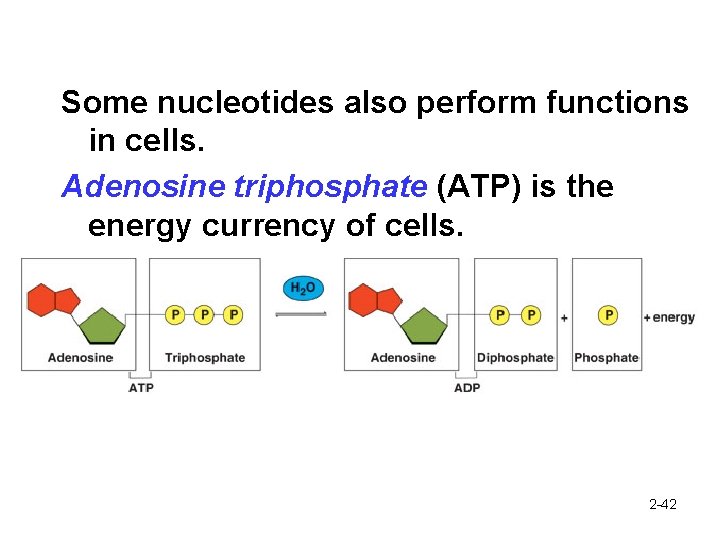
Some nucleotides also perform functions in cells. Adenosine triphosphate (ATP) is the energy currency of cells. 2 -42

2 -43
- Slides: 43