Processed Meat and Poultry Performance Standards Lethality and













- Slides: 15

Processed Meat and Poultry Performance Standards _________ Lethality and Stabilization Daniel Engeljohn USDA, FSIS May 9, 2001 1

Docket Information • Docket #97 -013 P • Performance Standards for the Production of Processed Meat and Poultry Products; Proposed Rule • 66 FR 12589, February 27, 2001 • Comment closing date extended; now due June 28, 2001 2

Proposed Definitions Relative to Lethality • 9 CFR 430. 1 • Fermented product – Made ready-to-eat by bacterial enzymes acting to lower p. H and microbial inhibition • Ready-to-eat product – Safe to consume without cooking or application of some other lethality treatment to destroy pathogens • Worst case product – Defined for lethality requirements in 9 CFR 430. 2(a)(1) for meat and poultry, and in 9 CFR 430. 2(b)(1) for beef 3

Proposed Text for Lethality: Salmonella • 9 CFR 430. 2(a) – Either a probability of survival • No greater than the specified Salmonella organisms in any 100 grams of finished product, assuming incoming product is worst case – Or a log reduction • 6. 5 -log 10 reduction for meat • 7. 0 -log 10 reduction for poultry – Detectable viable Salmonella adulterate ready-to -eat product 4

Proposed Text for Lethality: E. coli O 157: H 7 • 9 CFR 430. 2(b) – Either a probability of survival • No greater than the specified E. coli O 157: H 7 organisms in any 100 grams of finished (fermented beef) product, assuming incoming product is worse case – Or a log reduction • 5. 0 -log 10 reduction for fermented meat or poultry product containing beef – Detectable viable E. coli O 157: H 7 adulterate ready-to-eat product 5

Proposed Text for Lethality • 9 CFR 430. 2(c) – Reduction of other pathogens and their toxins or toxic metabolites: • Validated to prevent product adulteration • 9 CFR 430. 2(d) – Maintain the lethality performance standards throughout product shelf-life • Validated under the conditions in which the food is stored, distributed, and held 6

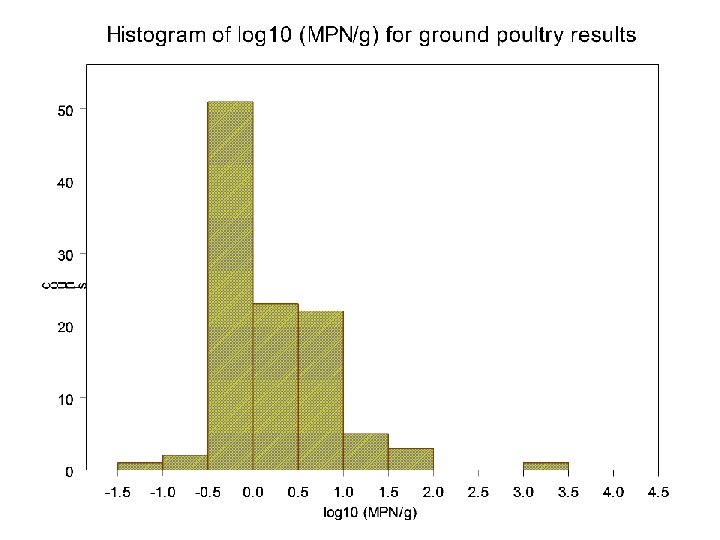
Determination of worst case levels: Lethality • Used highest Most-Probable-Number (MPN) results from FSIS national microbiology baseline surveys • MPN samples were frozen; samples were companion with those that tested Salmonella positive on a qualitative test • Conservative assumptions were made in determining worst case levels 7

8

Salmonella Lethality Performance Standard • Safety margin: – 0. 3 -log 10 added to worst case level resulting in the performance standard: • Poultry = 7. 0 -log 10 • Meat = 6. 5 -log 10 – Implies that after application of the lethality treatment on raw product containing worst case level of cells, probability of any viable cell is 39. 4% 9

E. coli O 157: H 7 Lethality Performance Standard for Fermented Beef • Safety margin: – 0. 6 -log 10 added to worst case level resulting in the performance standard: • Fermented beef = 5. 0 -log 10 – Implies that after application of the lethality treatment on fermented beef containing worst case level of cells, probability of any viable cell is 22. 2% 10

Proposed Text for Stabilization • 9 CFR 430. 3(a) – Processing of all ready-to-eat meat and poultry products must prevent multiplication of toxigenic microorganisms: • Clostridium botulinum • Allow no more than 1. 0 -log 10 multiplication of Clostridium perfringens • 9 CFR 430. 3(b) – Processing of all heat treated, not ready-toeat meat and poultry products must meet same criteria as in (a) above 11

Proposed Text for Stabilization • 9 CFR 430. 3(c) – Processing of products applicable to 9 CFR 430. 3(a) or (b) must be validated: • to maintain stabilization performance standards throughout product shelf-life under conditions in which the food is stored, distributed, and held 12

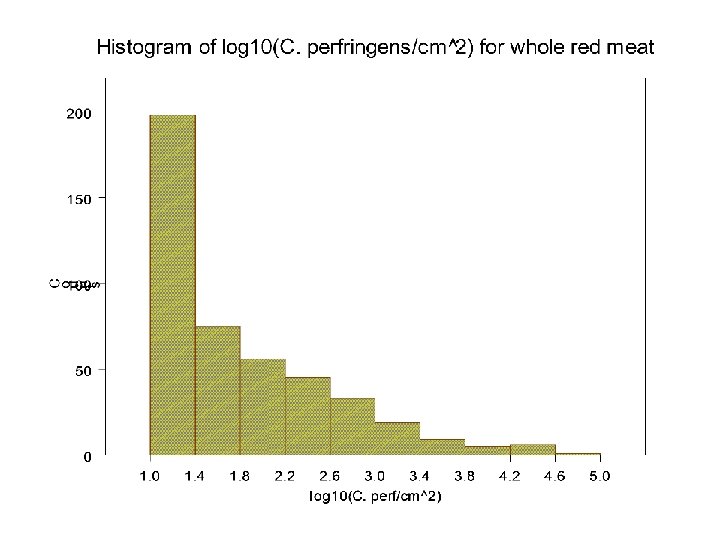
13

Determination of worst case level: C. perfringens • Used results from FSIS national microbiological baseline survey • No finding greater than 105/g • Worst case is assumed to be 104 C. perfringens per gram that become heat shocked, germinate, and after a lag period, multiply as vegetative cells during cooling 14

Draft Compliance Guidelines • Available now: – Constituent Update, May 4 – FSIS Website at: • www. fsis. usda. gov/OPPDE/rdad/FRPubs/RTEGuide. pdf • FSIS requests comment on these – Including suggestions for expansion to cover all types of ready-to-eat and partially heat-treated meat and poultry products • Draft guidelines – Not to be used for regulatory enforcement or compliance purposes 15