Process Validation For Oral Liquids Department of pharmaceutical











- Slides: 16

Process Validation For Oral Liquids Department of pharmaceutical science & Technology Birla institute of technology , Mesra Ranchi -835215( Jharkhand) 2019

After watching this video lecture we are going to LEARN: • What is Process Validation? • What are the different stages of Process Validation? • What are the types of Process Validation? • What are Oral Liquids? Classification? • Critical Parameter, Acceptance Criteria for the Validation of oral liquids. Process

Process Validation Establishing documented evidence which provide a high degree of assurance that a specific process with consistently produce a product meeting its predetermined specification and quality characteristics.

Objective Of Process Validation • • • Assuranace of quality of product. Decrease the risk of regulatory non compliance. Ensure the consistency of the manufacturing operation and reproducibility of the process. Easier maintenance of equipments. Improve employee awareness for process.

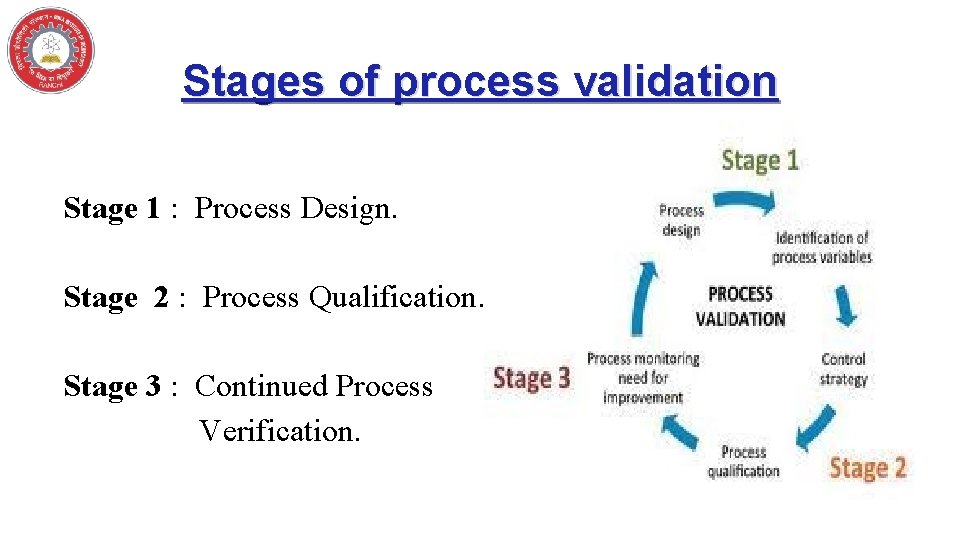
Stages of process validation Stage 1 : Process Design. Stage 2 : Process Qualification. Stage 3 : Continued Process Verification.

Types of process validation • Prospective : expected and expecting to be the specified things in future. • Concurrent : exiting , happening and done at the same time. • Retrospective : looking back on or dealing with past events or situations. • Revalidation: repetition of a validation process

Documentation in validation process • Validation master plan (VMP) • Validation protocol(VP) • Validation reports( VR) • Standard operating procedure (SOPs)

Now take a pause for a while to recall the topic which we have discussed befour, and try to answer the following questions: 1)what is process validation? 2)what are the different stages of process validation? 3)what are the types of process validation?

What are oral liquid ? Oral liquids are homogeneous liquid preparation, usually consisting of a solution an emulsion or a suspension of one ore more active ingredients in a suitable vehicle.

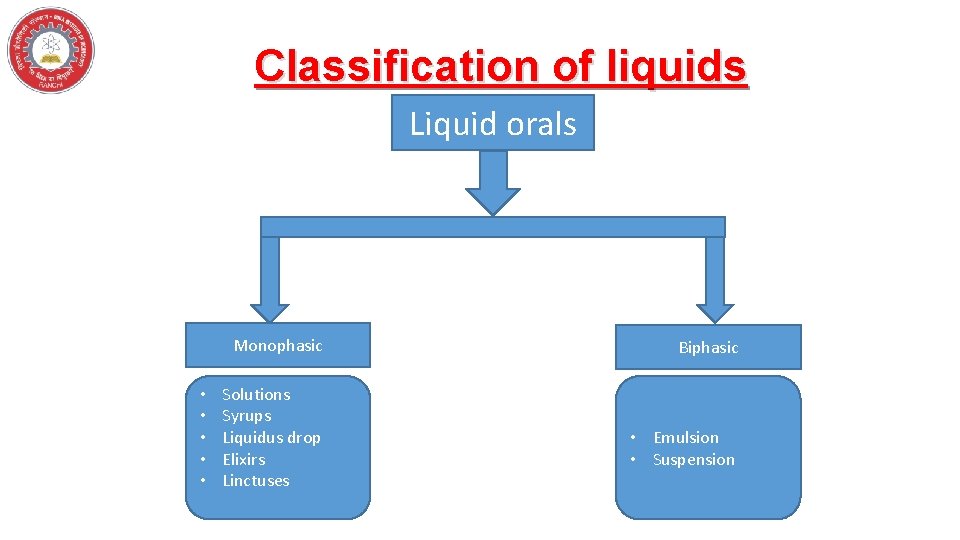
Classification of liquids Liquid orals Monophasic • • • Solutions Syrups Liquidus drop Elixirs Linctuses Biphasic • Emulsion • Suspension

Liquid dosage forms can be administrered • Topically • Orally ( per oral) • Parenterally ( S. c , I. M, I. V)

NUMBER OF VALIDATION TRIALS • FOR NEW PRODUCT , Product transfer or having major changes generally atleast three consecutive successful batches are required. SAMPLING FOR VALIDATION • FOR SOLUTION , Take atleast 2 samples at top and bottom of bulk. • FOR SUSPENSION, Take atleast 2 samples at top and bottom of bulk. • Finished product testing (net content , content uniformity)

VALIDATION REPORT • Report must be prepared and approved by Q. A. • Written notification and failure of process validation must be issued to top management. • In case of failure , an investigation must be completed and documented prior to repeat the validation study.

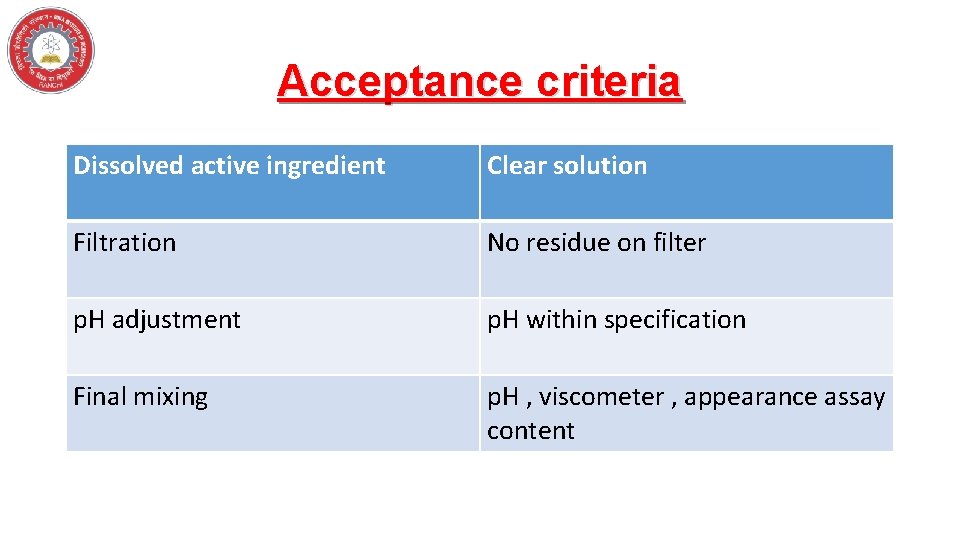
Acceptance criteria Dissolved active ingredient Clear solution Filtration No residue on filter p. H adjustment p. H within specification Final mixing p. H , viscometer , appearance assay content

CONCLUSION • Process validation is a proven assurance of the process efficiency and it is the fledged q. c tool for the pharmaceuticals industries. It eliminates the changes of batch failure as the products are manufactured as per pre optimization of each manufacturing steps.

Prepared by: Ms Riya Banerjee Ms Snigdha Baag Dr. Manik Ghosh Dr. Kishanta Kr. Pradhan Department of pharmaceutical science & Technology Birla institute of technology , Mesra Ranchi -835215( Jharkhand)