Process Development CHEE 2404 A Ghanem Process Development



























- Slides: 45

Process Development CHEE 2404 A Ghanem

Process Development • Development of new technologies and products is a Research and Development (R&D) activity • Goal: produce the desired product – – In time At planned rates At projected manufacturing cost At desired quality standards • Cost and planning are continually monitored from lab phase to production

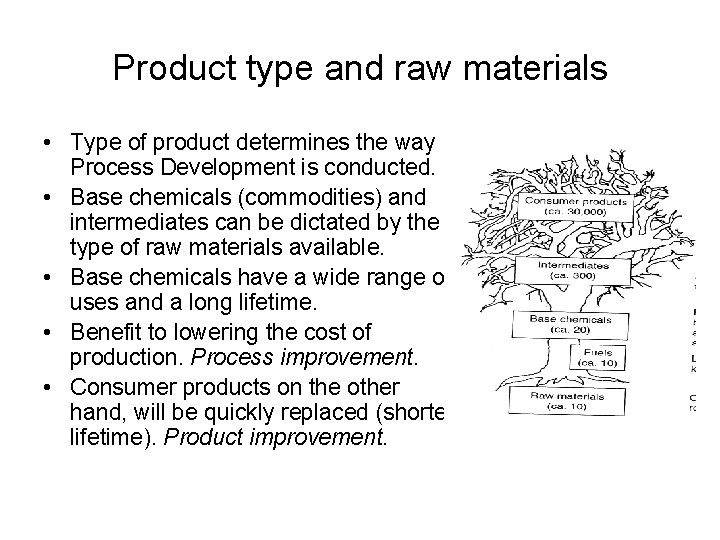
Product type and raw materials • Type of product determines the way Process Development is conducted. • Base chemicals (commodities) and intermediates can be dictated by the type of raw materials available. • Base chemicals have a wide range of uses and a long lifetime. • Benefit to lowering the cost of production. Process improvement. • Consumer products on the other hand, will be quickly replaced (shorter lifetime). Product improvement.

Base chemicals • The technologies for the production of base chemicals and intermediates are well established. • Development activities usually result in minor process improvements (example a new catalyst or a more energy efficient furnace) • Still can have a large impact on overall costs due to the large volumes involved (example, saving dollars per tonne of acetic acid can have a large impact when you produce 500, 000 t/a). • Major new advances in processes are still worth pursuing • More general drive is sustainability and process intensification

Consumer Products • In specialty chemicals, drive is toward modified or new products • Motivated by market demands rather than cost savings • Some market demands can include new products, product quality or environmental concerns. • Some examples are environmentally friendly paints, varied detergents, wood composites used in building materials, new drugs, etc. • More effort is required in determining the chemical route of manufacturing. For example, a when a new drug is approved the manufacturing route must also be approved.

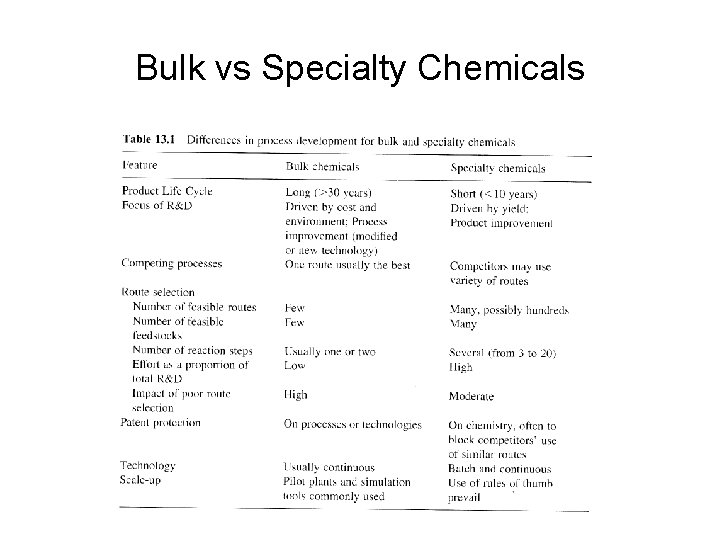
Bulk vs Specialty Chemicals

Process Development • Continuous interaction between experimentation and economics. • Input: chemical reaction in the lab • Outcome: production plant • Development of a process to take the chemical reaction in the lab up to a large plant scale in an economic way. • Enlargement of equipment in small steps? Empirical method, and practical for some applications but not generally for continuous process.

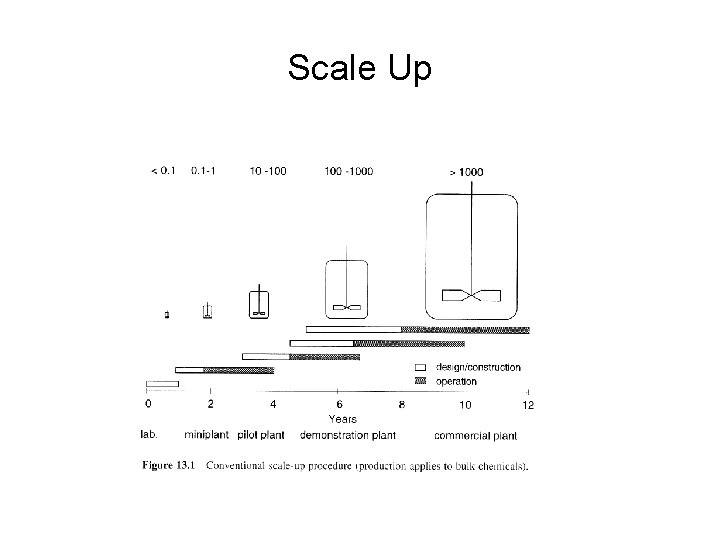
Scale Up

• Scale up in small steps is expensive especially for larger continuous production plants. • Large safety margins are used. • Time scale shown is very long (8 years. . . need to reduce time to process development) due to time associated with design/construction and operation of small steps. • Predictive models: Process steps described in a Mathematical model with predictive value • Predictive models are used to scale up equipment and processes from laboratory data or pilot plant to eliminate steps and save time.

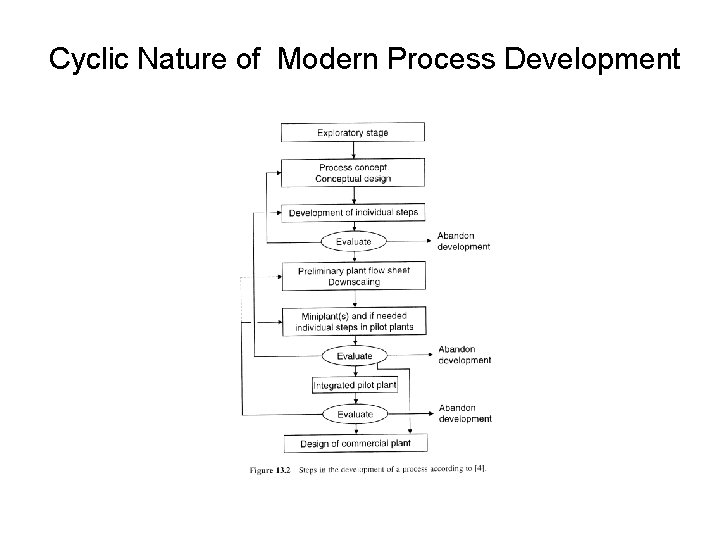
• Exploratory phase: the reaction provides satisfactory yields. • Based on lab data and literature data, the process concept is put together. • Individual steps are developed and tested on a lab scale (the reactor, does the required separation work? ) • A process flow sheet is drawn. • A small scale plant is designed (mini-plant) to evaluate performance of the entire process.

• A pilot plant may be designed and built for further testing. • At each stage, evaluation occurs. Continue, stop, or go back to an earlier stage? • Decisions are based on technical, cost and market considerations.

Cyclic Nature of Modern Process Development

Breakdown of Steps • Exploratory Phase – Discovery of a new product, a new chemical synthesis route, or an improvement to a process – Focus on chemical reaction – Obtain information: • what reactions take place • thermodynamics and kinetics of the reaction • selectivity and conversion rates and their dependence on process parameters • Catalyst and catalyst deactivation rate

Breakdown of Steps • Preliminary Flowsheet – Determine the availability and quality of raw materials (first design step would be compare raw material costs with product value) – Draw up preliminary flowsheets and alternatives – Typically underdefined, so we must make assumptions. – What units should be used? – How will the units be connected? – What T, P and flowrates will be required? – Difficult b/c there are many ways we can accomplish the goal, problem is open ended.

• Process requirements: – Lowest cost – Satisfies environmental constraints – Easy to start up and operate • We can eliminate alternatives based on the above considerations • Make the optimal choice based on knowledge, experience and tools such as process simulation

Input Information • • • The reactions and reaction conditions Desired production rate Desired product purity ($ vs. purity) Raw materials (also need $ vs. purity info here) Information on rate of reaction, catalyst deactivation Processing constraints? (ex. explosion limits, conditions that cause polymerization, etc. ) Plant and Site data Physical properties of all components Information on safety, toxicity, environmental impact of materials involved in the process. Cost data for byproducts, equipment and utilities.

1. Reactor system… 2. Separations • Reactor System – First step – Includes reactor, feed, product gas and liquid recycle streams. – Influences the yields, product distribution and separations. – example: coal gasification, the amount of H 2 and CO 2 formed are very different for a moving bed, fluidized bed, and entrained flow reactor. – Determine T and P, type of catalyst, phases of reactant and products

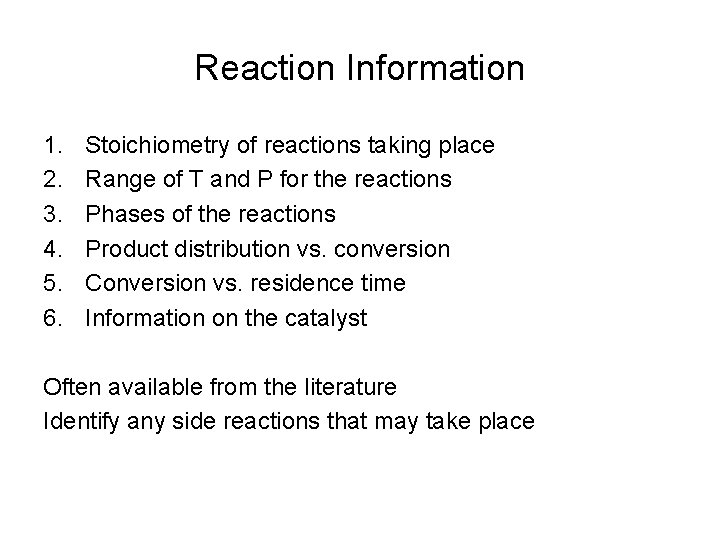
Reaction Information 1. 2. 3. 4. 5. 6. Stoichiometry of reactions taking place Range of T and P for the reactions Phases of the reactions Product distribution vs. conversion Conversion vs. residence time Information on the catalyst Often available from the literature Identify any side reactions that may take place

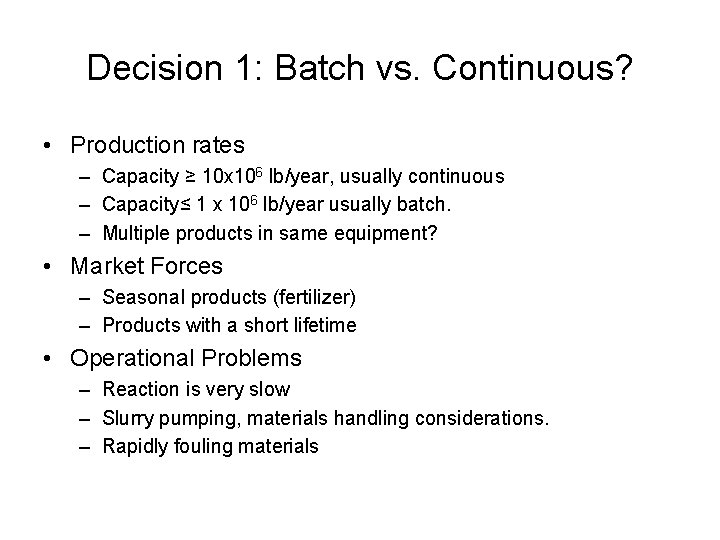
Decision 1: Batch vs. Continuous? • Production rates – Capacity ≥ 10 x 106 lb/year, usually continuous – Capacity≤ 1 x 106 lb/year usually batch. – Multiple products in same equipment? • Market Forces – Seasonal products (fertilizer) – Products with a short lifetime • Operational Problems – Reaction is very slow – Slurry pumping, materials handling considerations. – Rapidly fouling materials

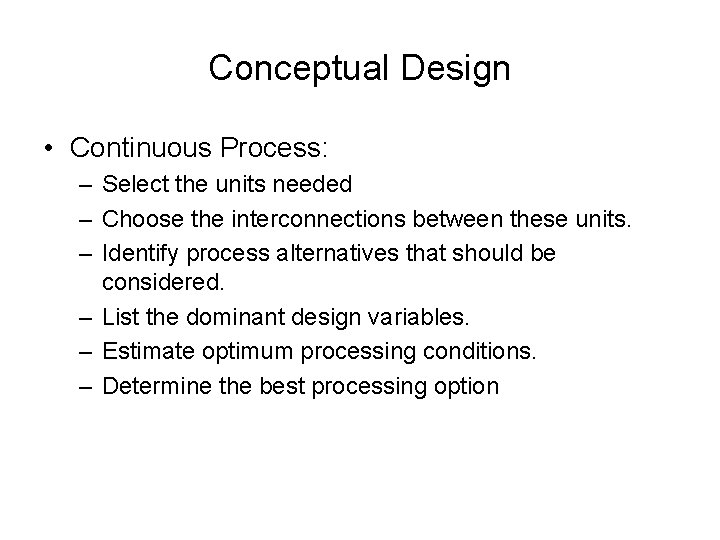
Conceptual Design • Continuous Process: – Select the units needed – Choose the interconnections between these units. – Identify process alternatives that should be considered. – List the dominant design variables. – Estimate optimum processing conditions. – Determine the best processing option

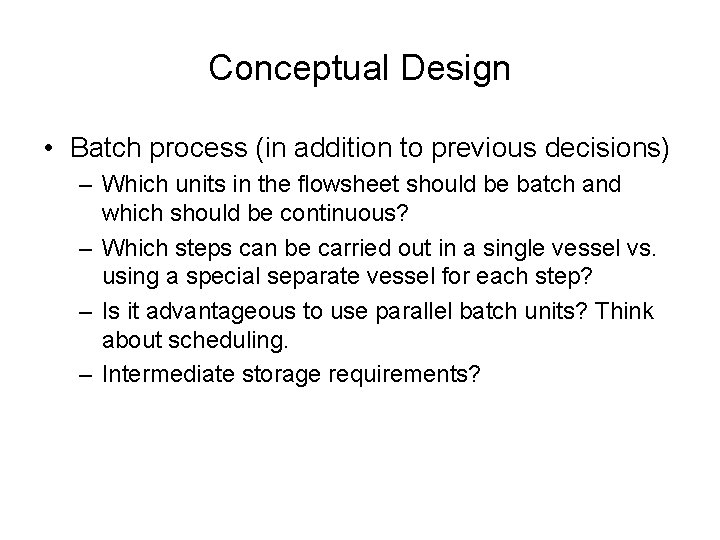
Conceptual Design • Batch process (in addition to previous decisions) – Which units in the flowsheet should be batch and which should be continuous? – Which steps can be carried out in a single vessel vs. using a special separate vessel for each step? – Is it advantageous to use parallel batch units? Think about scheduling. – Intermediate storage requirements?

Decision 2: inputs and outputs • Should you purify the feed streams before they enter the process? • Should you remove or recycle a by product? • Should you use a gas recycle or purge stream? • Should you recycle unreacted reactants? • How many product streams will there be?

Decision 3: Separations – Reaction product contains multiple components, you must decide how they will be separated and at what conditions. – Look at the components and how they differ (ie boiling point, solubility) – Identify possible unit operations (ie. Distillation, absorption, adsorption, solvent extraction, etc. ) – If reactor effluent is a liquid, use liquid separation system • distillation • liquid extraction, etc. • Avoid gas absorbers, gas adsorbers.

– If reactor effluent is a 2 phase mixture, send liquid to a liquid system, cool the vapour and send to vapour recovery – – Condenser Absorption Adsorption membrane separations). – If either stream has reactants, recycle these. – Reactor effluent all vapour – cool to attempt to condense liquid. Follow up by sending vapour to vapour recovery, liquid to liquid recovery.

Heuristic rules for distillation sequence • Remove corrosive or hazardous components as soon as possible • Remove reactive components or monomers as soon as possible • Remove products as distillates • Remove recycles as distillates, particularly if they are recycled to a fixed bed reactor • Remove most plentiful first • Remove Lightest first • High recovery separation last • Difficult separation last

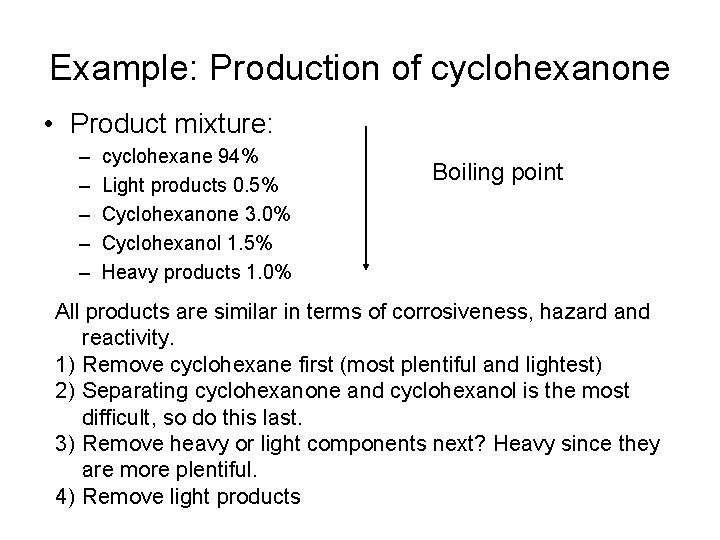
Example: Production of cyclohexanone • Product mixture: – – – cyclohexane 94% Light products 0. 5% Cyclohexanone 3. 0% Cyclohexanol 1. 5% Heavy products 1. 0% Boiling point All products are similar in terms of corrosiveness, hazard and reactivity. 1) Remove cyclohexane first (most plentiful and lightest) 2) Separating cyclohexanone and cyclohexanol is the most difficult, so do this last. 3) Remove heavy or light components next? Heavy since they are more plentiful. 4) Remove light products

• Draw the flowsheet of selected operations • Sizing of unit operations is done based on available information at that time (this is preliminary as not all data is available yet, an iterative process). • Develop and test the individual steps. • Laboratory tests conducted on mixtures prepared from pure materials, typically short duration, may not show some problems. • Pilot plants are the next step

Pilot Plants • An experimental system that represents the part it corresponds to in an industrial unit. • Can range in size from lab scale (mini plant) to commercial unit (demonstration plant). • Used to : – – – generate more product to develop a market confirm feasibility of the process check design calculations solve scaleup problems on novel processes gain operational know-how Determine long term effects of operation • Typically run to 10% of the commercial plant cost.

Mini-plant • To demonstrate process feasibility or generate design data for a process, then a mini plant may be more appropriate than a pilot plant. • Includes all recycle streams and can be extrapolated reliably • Uses same components as the lab testing (ie pumps, etc. ), which is often standardized and can be used in many other mini plants • Operated continuously for weeks or months so some automation is required. • Is used in combination with process modeling and simulation of the industrial scale process. • Typically produces 0. 1 -1 kg product per hour.

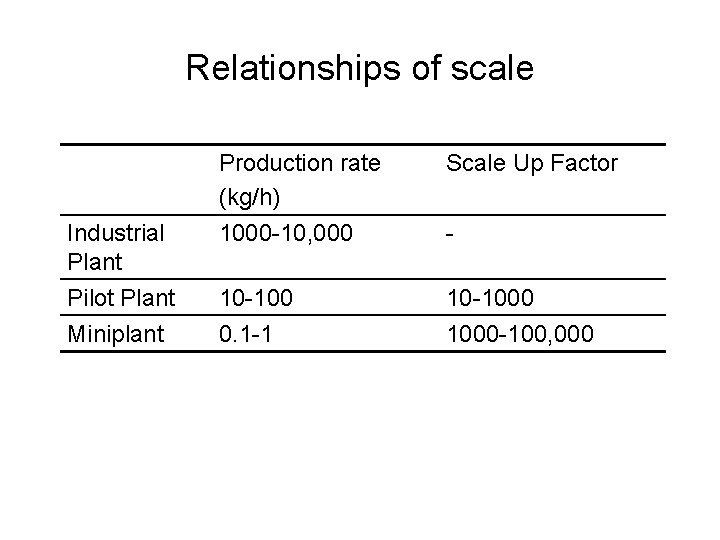
Relationships of scale Industrial Plant Pilot Plant Miniplant Production rate (kg/h) 1000 -10, 000 Scale Up Factor 10 -100 0. 1 -1 10 -1000 -100, 000 -

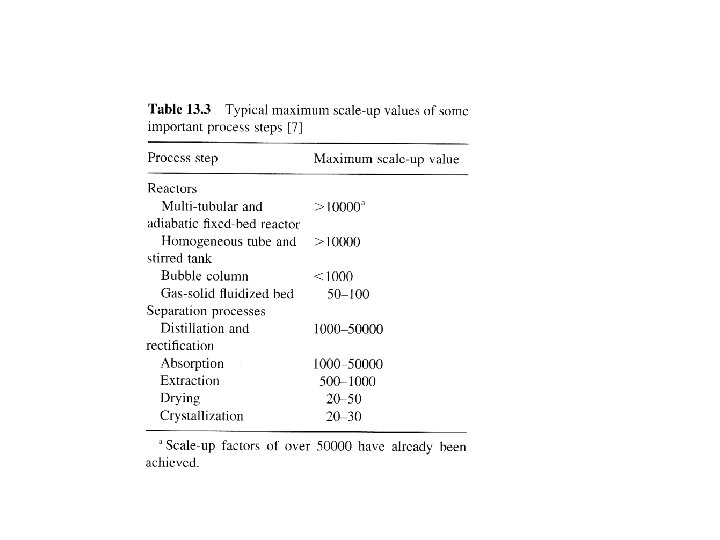
Miniplants • Can help to speed up process development and at a lower cost. • Useful to test catalyst stability under practical conditions. • Incorporate recycle streams to detect buildup and effect of impurities. • Some unit operations not easily scaled from miniplant data (extraction, crystallization, fluidized beds) due to flow characteristics. • See fig 13. 3 for some scaleup values


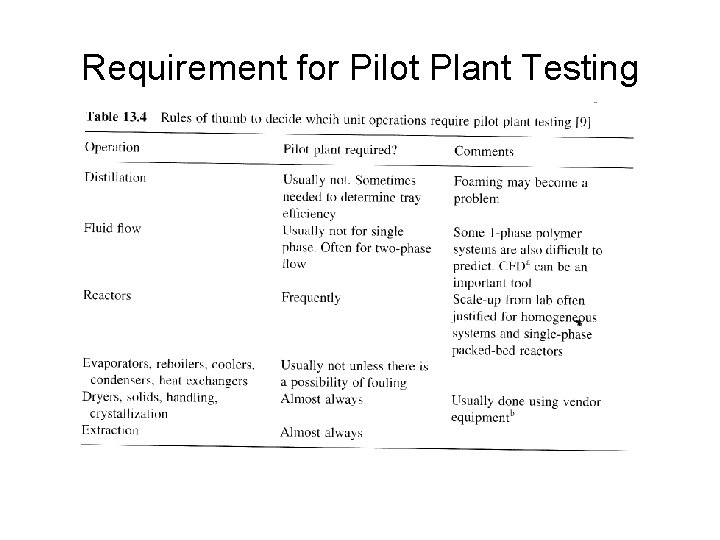
Requirement for Pilot Plant Testing

Reactor Scale Up • Homogeneous Reactors (single fluid phase) • Easier to scale up than heterogeneous • Batch or semi-batch reactor – Main issue is heat removal in highly exothermic reactions • Continuous tubular reactor – Main issue is heat transfer and T profile in the reactor, kinetic modeling of reactions is used to relate the reaction to temperature • Continuous stirred tank reactor – Scale up from batch reactor kinetic data

Heterogeneous Reactors • Examples include steam reforming, ammonia synthesis, hydrotreating. • Main issues are T control, P drop and Catalyst deactivation • Temperature control – In endothermic reactions the T drop may be severe resulting in an excessively long reactor – Reaction mixture must be heated rapidly to keep the reaction rate at a high enough level – One way of doing this is to conduct reaction in tubes in a furnace (steam reforming)

• Temperature control – Exothermic reactions need to be cooled – This can be done by external heat exchangers, injection of cold feed gas. • Pressure Drop – Pressure drop across a catalyst bed must be limited – Reduce the bed height, use larger particles, apply axial flow or structure the reactor. • Catalyst deactivation – Design strategies depend on the mechanism of catalyst deactivation

• Catalyst deactivation – For example, if the catalyst is deactivated by coke deposits, regeneration occurs by burning off coke. This can be done in a fluid bed reactor. – Impurities may build up in the system that are undetected at lab scale (low concentration), that may affect the catalyst if they are recycled. Larger scale reactions are needed to detect these so processes can be established to deal with them. – Install pretreatment units, purge some of the recycle stream.

• Hyrodynamics – Fluid distribution in a heterogeneous reactor may change as you make a reactor larger. – Gas-liquid, solid-liquid contacting – Parameters include diameter and height, residence time, catalyst particle size

Safety and Loss Prevention • Chemical plants involve process, storage and transport of hazardous materials. • Increasing plant size increases risks • Plants are often located close to dense populations. • Loss prevention: identify the hazards of a chemical process plant and eliminate them. • Major hazards: – Explosion – Fire – Release of toxic substance


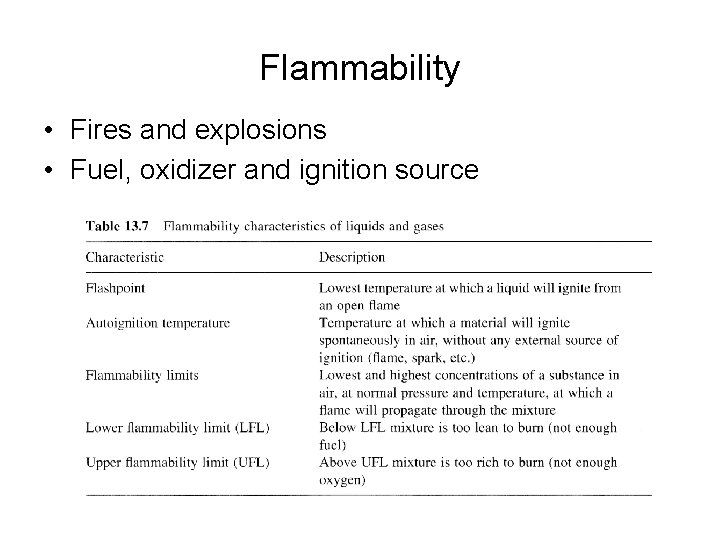
Flammability • Fires and explosions • Fuel, oxidizer and ignition source


Toxicity “The dose makes the poison” • • • Hazard depends on the inherent toxicity Frequency and duration of exposure Acute vs. chronic effects LD 50: lethal dose that kills 50% of test animals TLV: threshold limit value, conc of exposure for 8 hours a day, 5 days a week, without harm. • Strategies: substitution, containment, ventilation, disposal provisions, Good manufacturing practices (GMP)

Reactivity Hazards • Exothermic runaway reactions • Reactions can occur anywhere • Unused Catalysts may mediate undesired reactions • Have good knowledge of reaction chemistry and reaction enthalpies • Use of nitrogen blanket to keep systems inert

Process Evaluation Evaluate at each stage of development • Is the process technically feasible? – This is determined at the laboratory, flowsheet design, and pilot plant level • Is it economically attractive? • How big is the risk (economically, technically)?