Problem A melt or water solution that a


Problem: • A melt or water solution that a mineral precipitates from contains ALL natural elements • Question: Do any of these ‘other’ ions get into a particular mineral?

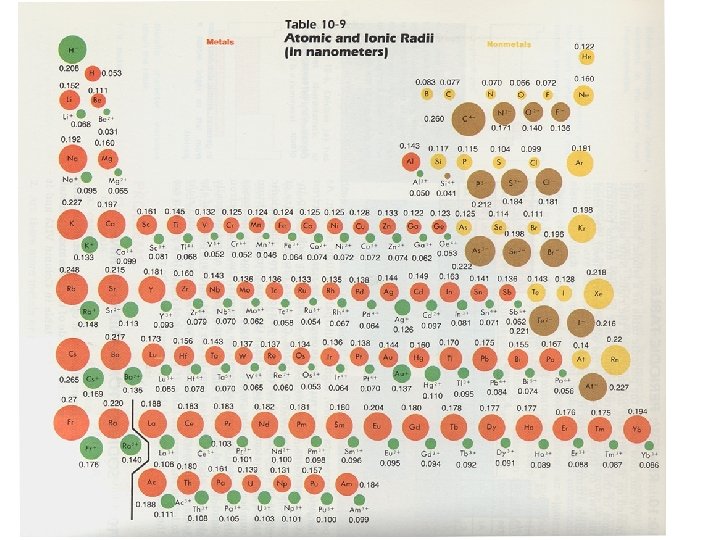
Goldschmidt’s rules of Substitution 1. The ions of one element can extensively replace those of another in ionic crystals if their radii differ by less than about 15% 2. Ions whose charges differ by one may substitute readily if electrical neutrality is maintained – if charge differs by more than one, substitution is minimal
Goldschmidt’s rules of Substitution 3. When 2 ions can occupy a particular position in a lattice, the ion with the higher charge density forms a stronger bond with the anions surrounding the site 4. Substitution may be limited when the electronegativities of competing ions are different, forming bonds of different ionic character
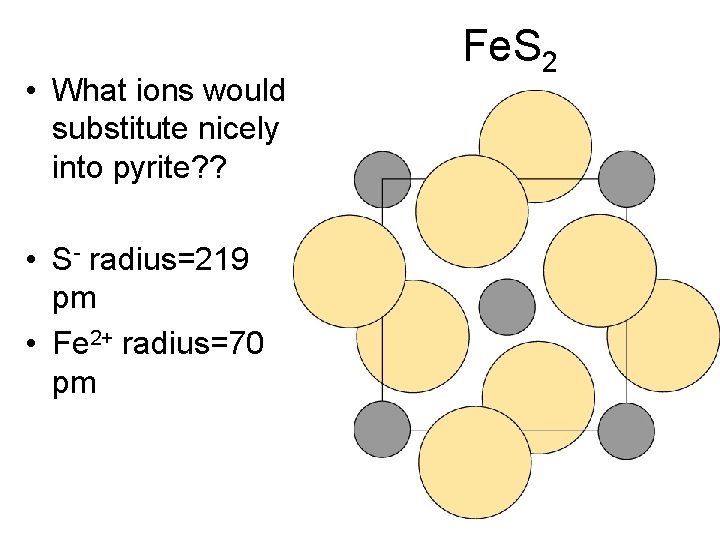
• What ions would substitute nicely into pyrite? ? • S- radius=219 pm • Fe 2+ radius=70 pm Fe. S 2
Chemical ‘fingerprints’ of minerals • Major, minor, and trace constituents in a mineral • Stable isotopic signatures • Radioactive isotope signatures

Major, minor, and trace constituents in a mineral • A handsample-size rock or mineral has around 5*1024 atoms in it – theoretically almost every known element is somewhere in that rock, most in concentrations too small to measure… • Specific chemical composition of any mineral is a record of the melt or solution it precipitated from. Exact chemical composition of any mineral is a fingerprint, or a genetic record, much like your own DNA • This composition may be further affected by other processes • Can indicate provenance (origin), and from looking at changes in chemistry across adjacant/similar units - rate of precipitation/ crystallization, melt history, fluid history
Chemical heterogeneity • Matrix containing ions a mineral forms in contains many different ions/elements – sometimes they get into the mineral • Ease with which they do this: – Solid solution: ions which substitute easily form a series of minerals with varying compositions (olivine series how easily Mg (forsterite) and Fe (fayalite) swap…) – Impurity defect: ions of lower quantity or that have a harder time swapping get into the structure
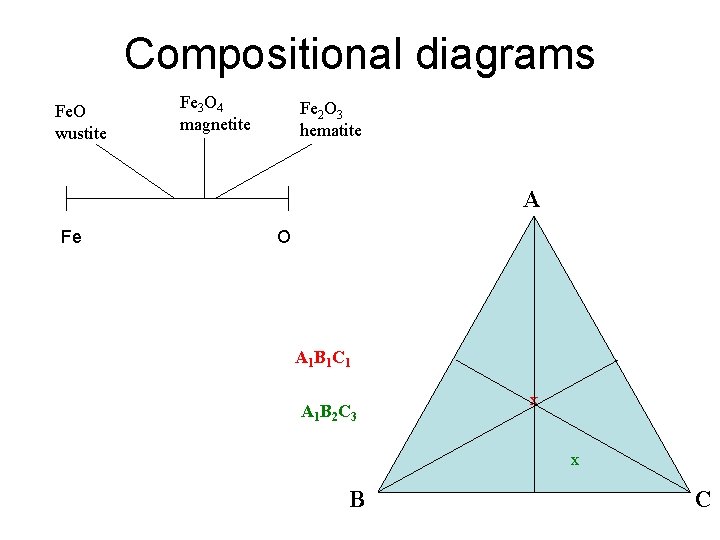
Compositional diagrams Fe. O wustite Fe 3 O 4 magnetite Fe 2 O 3 hematite A Fe O A 1 B 1 C 1 A 1 B 2 C 3 x x B C
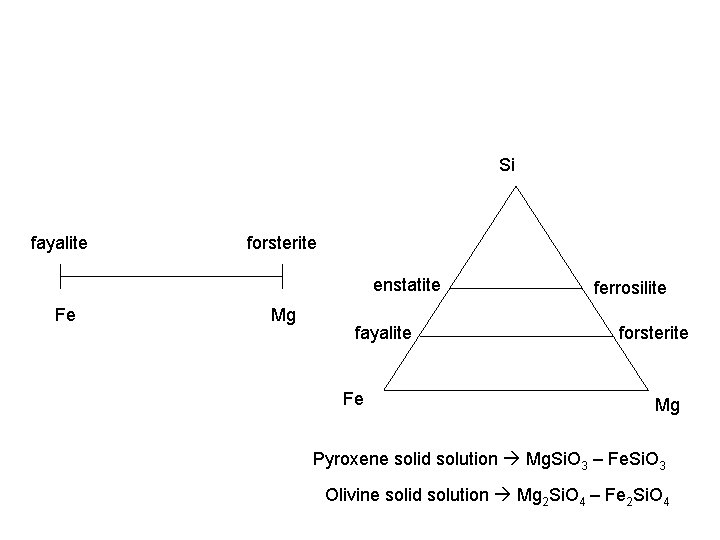
Si fayalite forsterite enstatite Fe Mg fayalite Fe ferrosilite forsterite Mg Pyroxene solid solution Mg. Si. O 3 – Fe. Si. O 3 Olivine solid solution Mg 2 Si. O 4 – Fe 2 Si. O 4
Stable Isotopes • A number of elements have more than one naturally occuring stable isotope. – Why atomic mass numbers are not whole they represent the relative fractions of naturally occurring stable isotopes • Any reaction involving one of these isotopes can have a fractionation – where one isotope is favored over another • Studying this fractionation yields information about the interaction of water and a mineral/rock, the origin of O in minerals, rates of weathering, climate history, and details of magma evolution, among other processes

Radioactive Isotopes • Many elements also have 1+ radioactive isotopes • A radioactive isotope is inherently unstable and through radiactive decay, turns into other isotopes (a string of these reactions is a decay chain) • The rates of each decay are variable – some are extremely slow • If a system is closed (no elements escape) then the proportion of parent (original) and daughter (product of a radioactive decay reaction) can yield a date. • Radioactive isotopes are also used to study petrogenesis, weathering rates, water/rock interaction, among other processes
- Slides: 13