Probability Calculations To calculate the probability of finding
Probability Calculations
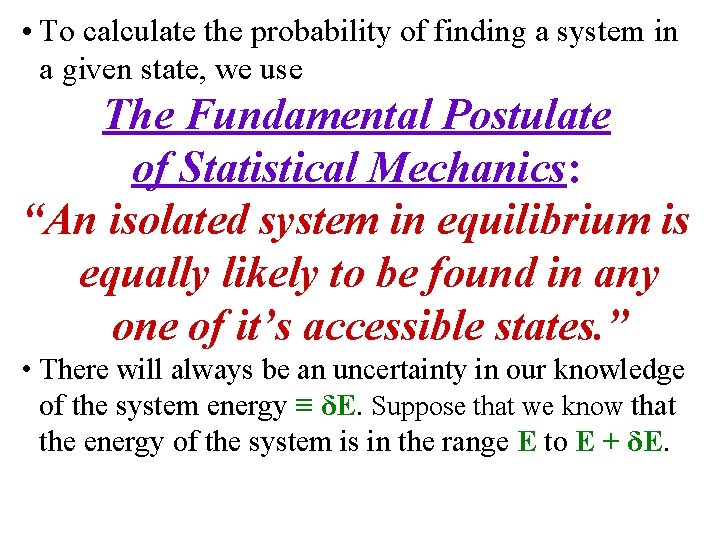
• To calculate the probability of finding a system in a given state, we use The Fundamental Postulate of Statistical Mechanics: “An isolated system in equilibrium is equally likely to be found in any one of it’s accessible states. ” • There will always be an uncertainty in our knowledge of the system energy ≡ δE. Suppose that we know that the energy of the system is in the range E to E + δE.
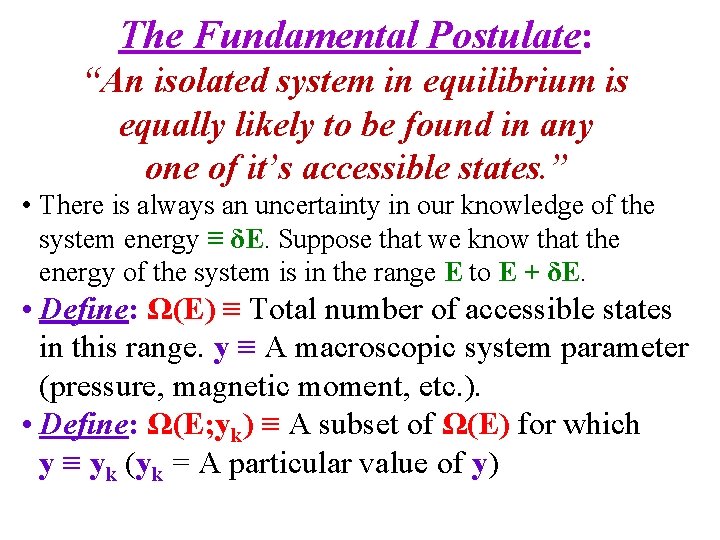
The Fundamental Postulate: “An isolated system in equilibrium is equally likely to be found in any one of it’s accessible states. ” • There is always an uncertainty in our knowledge of the system energy ≡ δE. Suppose that we know that the energy of the system is in the range E to E + δE. • Define: Ω(E) ≡ Total number of accessible states in this range. y ≡ A macroscopic system parameter (pressure, magnetic moment, etc. ). • Define: Ω(E; yk) ≡ A subset of Ω(E) for which y ≡ yk (yk = A particular value of y)
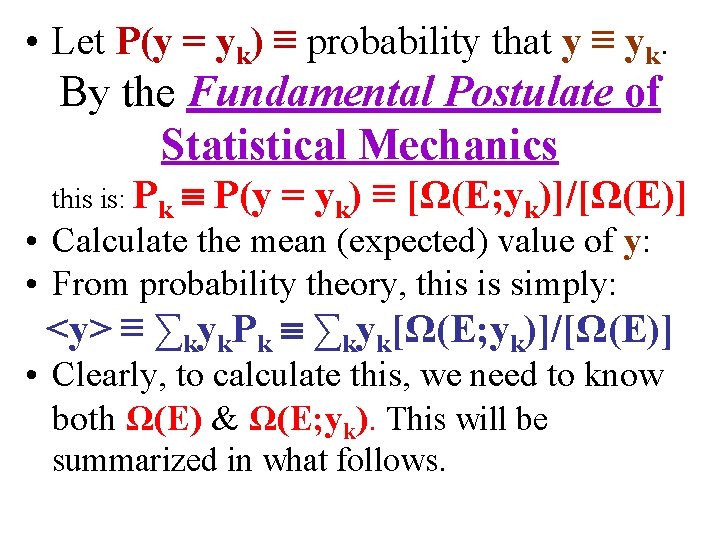
• Let P(y = yk) ≡ probability that y ≡ yk. By the Fundamental Postulate of Statistical Mechanics this is: Pk P(y = yk) ≡ [Ω(E; yk)]/[Ω(E)] • Calculate the mean (expected) value of y: • From probability theory, this is simply: <y> ≡ ∑kyk. Pk ∑kyk[Ω(E; yk)]/[Ω(E)] • Clearly, to calculate this, we need to know both Ω(E) & Ω(E; yk). This will be summarized in what follows.
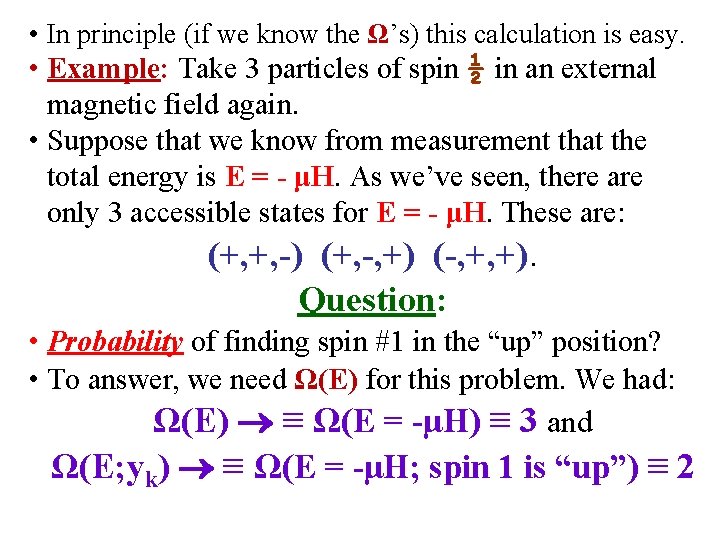
• In principle (if we know the Ω’s) this calculation is easy. • Example: Take 3 particles of spin ½ in an external magnetic field again. • Suppose that we know from measurement that the total energy is E = - μH. As we’ve seen, there are only 3 accessible states for E = - μH. These are: (+, +, -) (+, -, +) (-, +, +). Question: • Probability of finding spin #1 in the “up” position? • To answer, we need Ω(E) for this problem. We had: Ω(E) ≡ Ω(E = -μH) ≡ 3 and Ω(E; yk) ≡ Ω(E = -μH; spin 1 is “up”) ≡ 2
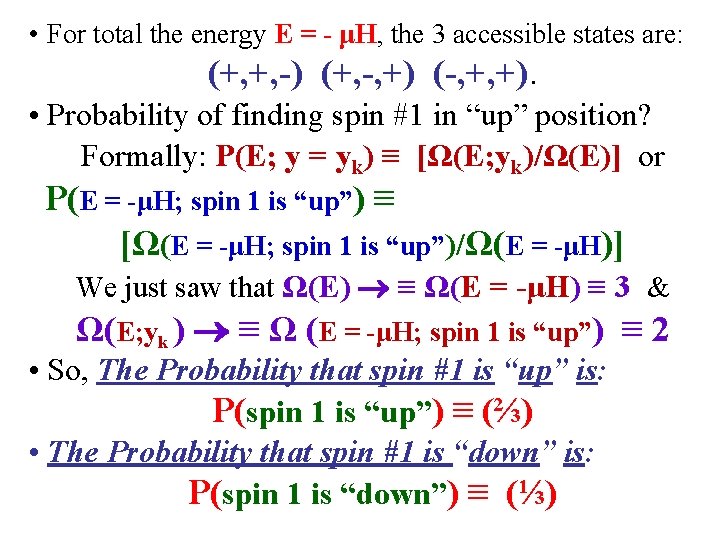
• For total the energy E = - μH, the 3 accessible states are: (+, +, -) (+, -, +) (-, +, +). • Probability of finding spin #1 in “up” position? Formally: P(E; y = yk) ≡ [Ω(E; yk)/Ω(E)] or P(E = -μH; spin 1 is “up”) ≡ [Ω(E = -μH; spin 1 is “up”)/Ω(E = -μH)] We just saw that Ω(E) ≡ Ω(E = -μH) ≡ 3 & Ω(E; yk ) ≡ Ω (E = -μH; spin 1 is “up”) ≡ 2 • So, The Probability that spin #1 is “up” is: P(spin 1 is “up”) ≡ (⅔) • The Probability that spin #1 is “down” is: P(spin 1 is “down”) ≡ (⅓)
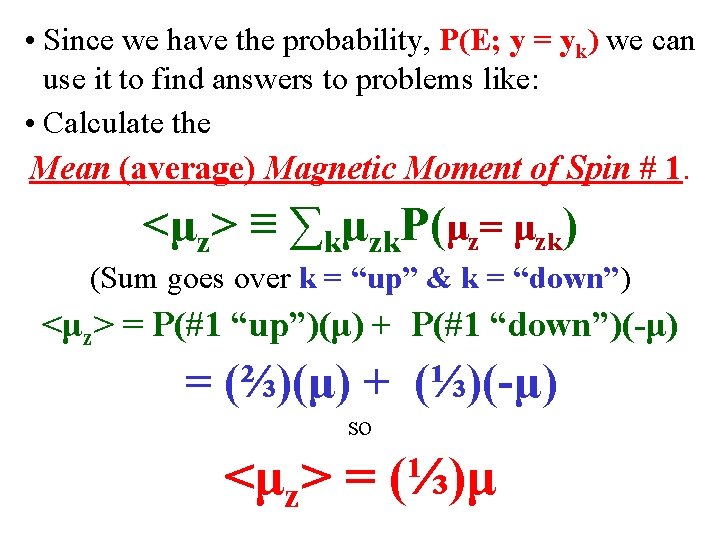
• Since we have the probability, P(E; y = yk) we can use it to find answers to problems like: • Calculate the Mean (average) Magnetic Moment of Spin # 1. <μz> ≡ ∑kμzk. P(μz= μzk) (Sum goes over k = “up” & k = “down”) <μz> = P(#1 “up”)(μ) + P(#1 “down”)(-μ) = (⅔)(μ) + (⅓)(-μ) so <μz> = (⅓)μ

Energy Dependence of the Density of States
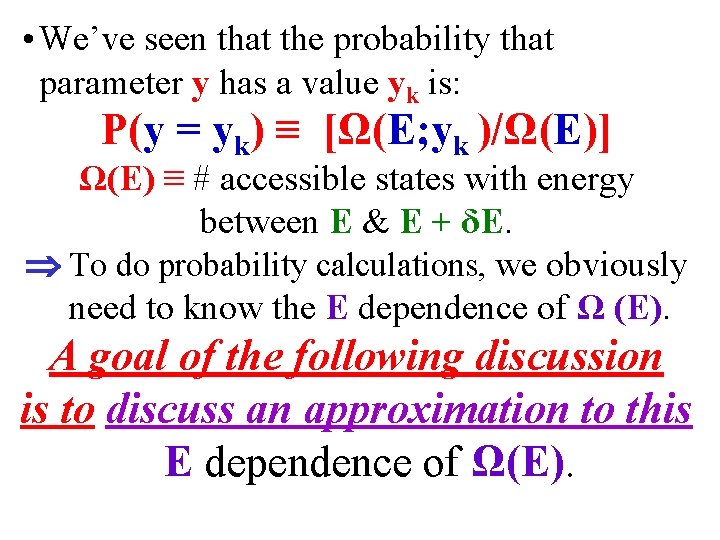
• We’ve seen that the probability that parameter y has a value yk is: P(y = yk) ≡ [Ω(E; yk )/Ω(E)] Ω(E) ≡ # accessible states with energy between E & E + δE. To do probability calculations, we obviously need to know the E dependence of Ω (E). A goal of the following discussion is to discuss an approximation to this E dependence of Ω(E).
![P(y = yk) ≡ [Ω(E; yk)/Ω(E)] Discussion of an approximation for the E dependence P(y = yk) ≡ [Ω(E; yk)/Ω(E)] Discussion of an approximation for the E dependence](http://slidetodoc.com/presentation_image/9924093585ee01bd780b8bffa9cefef6/image-10.jpg)
P(y = yk) ≡ [Ω(E; yk)/Ω(E)] Discussion of an approximation for the E dependence of Ω(E). • No rigorous math! Physical arguments & “hand waving. ” • Math details are in many books! Consider a Macroscopic System with f degrees of freedom. f is huge! f ~ 1024. • Energy is in range E to E + δE. Of course, Ω(E) δE. Extrapolating earlier discussion of the 1 d oscillator: Ω(E) ~ Phase space volume in this energy range. • Define Ω(E) ≡ ω(E)δE. ω(E) ≡ Density of states
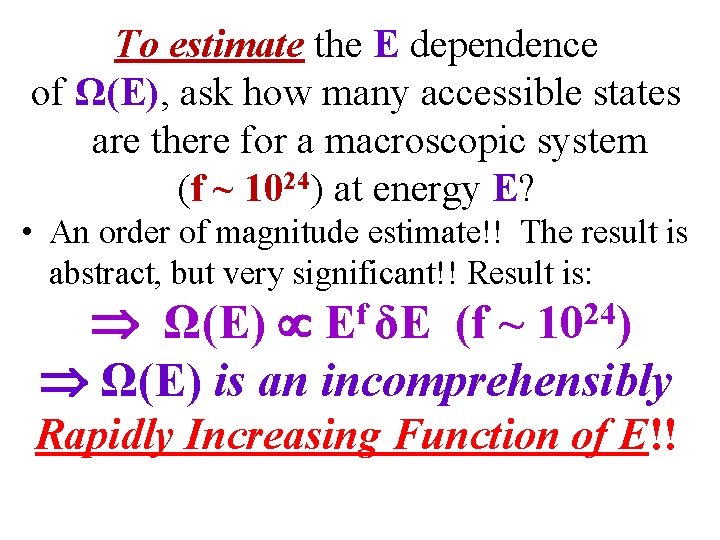
To estimate the E dependence of Ω(E), ask how many accessible states are there for a macroscopic system (f ~ 1024) at energy E? • An order of magnitude estimate!! The result is abstract, but very significant!! Result is: Ω(E) (f ~ Ω(E) is an incomprehensibly f E δE 24 10 ) Rapidly Increasing Function of E!!
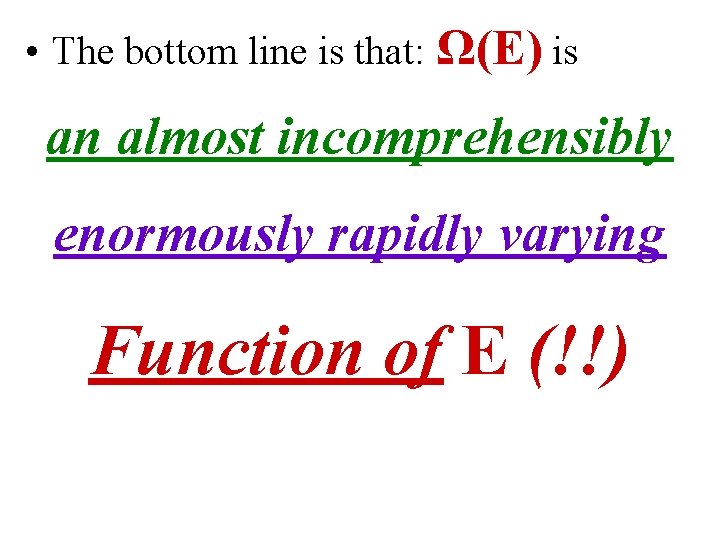
• The bottom line is that: Ω(E) is an almost incomprehensibly enormously rapidly varying Function of E (!!)
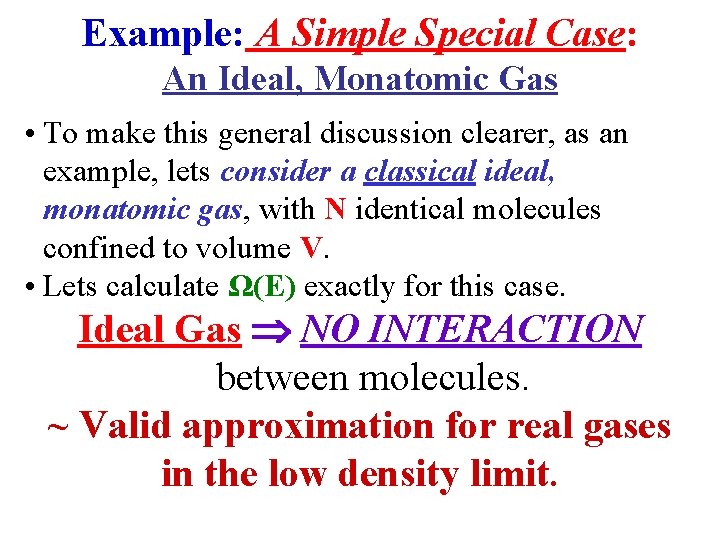
Example: A Simple Special Case: An Ideal, Monatomic Gas • To make this general discussion clearer, as an example, lets consider a classical ideal, monatomic gas, with N identical molecules confined to volume V. • Lets calculate Ω(E) exactly for this case. Ideal Gas NO INTERACTION between molecules. ~ Valid approximation for real gases in the low density limit.
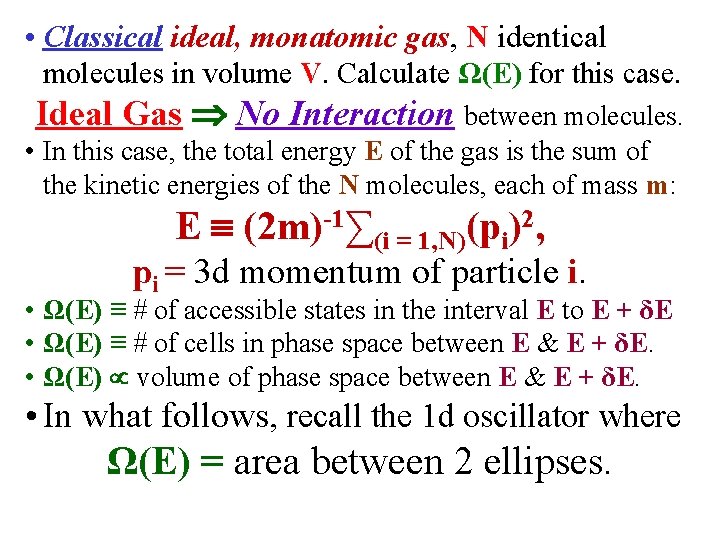
• Classical ideal, monatomic gas, N identical molecules in volume V. Calculate Ω(E) for this case. Ideal Gas No Interaction between molecules. • In this case, the total energy E of the gas is the sum of the kinetic energies of the N molecules, each of mass m: E (2 m)-1∑(i = 1, N)(pi)2, pi = 3 d momentum of particle i. • Ω(E) ≡ # of accessible states in the interval E to E + δE • Ω(E) ≡ # of cells in phase space between E & E + δE. • Ω(E) volume of phase space between E & E + δE. • In what follows, recall the 1 d oscillator where Ω(E) = area between 2 ellipses.
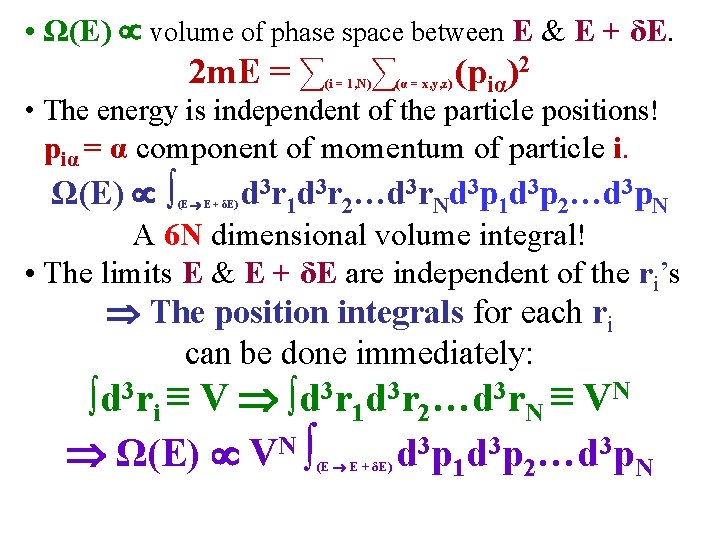
• Ω(E) volume of phase space between E & E + δE. 2 m. E = ∑ ∑ (i = 1, N) (α = x, y, z) (piα)2 • The energy is independent of the particle positions! piα = α component of momentum of particle i. Ω(E) ∫ (E E + δE) d 3 r 1 d 3 r 2…d 3 r. Nd 3 p 1 d 3 p 2…d 3 p. N A 6 N dimensional volume integral! • The limits E & E + δE are independent of the ri’s The position integrals for each ri can be done immediately: ∫d 3 ri ≡ V ∫d 3 r 1 d 3 r 2…d 3 r. N ≡ VN Ω(E) VN ∫ d 3 p 1 d 3 p 2…d 3 p. N (E E + δE)
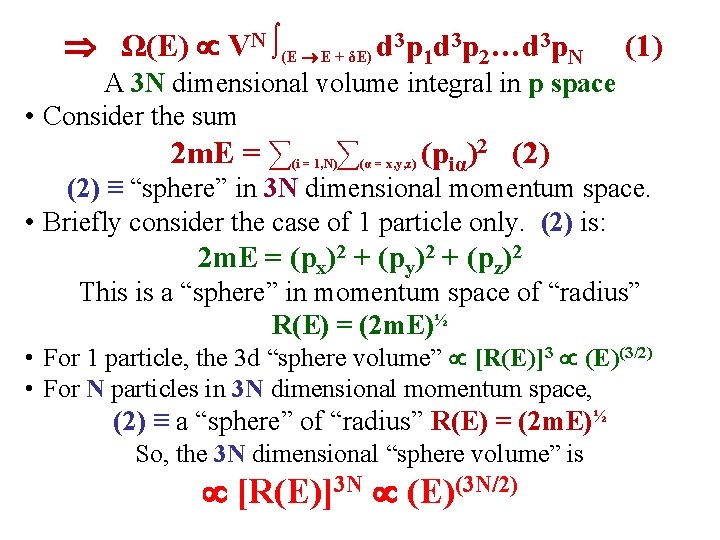
Ω(E) VN ∫(E E + δE) d 3 p 1 d 3 p 2…d 3 p. N (1) A 3 N dimensional volume integral in p space • Consider the sum 2 m. E = ∑(i = 1, N)∑(α = x, y, z) (piα)2 (2) ≡ “sphere” in 3 N dimensional momentum space. • Briefly consider the case of 1 particle only. (2) is: 2 m. E = (px)2 + (py)2 + (pz)2 This is a “sphere” in momentum space of “radius” R(E) = (2 m. E)½ • For 1 particle, the 3 d “sphere volume” [R(E)]3 (E)(3/2) • For N particles in 3 N dimensional momentum space, (2) ≡ a “sphere” of “radius” R(E) = (2 m. E)½ So, the 3 N dimensional “sphere volume” is [R(E)]3 N (E)(3 N/2)
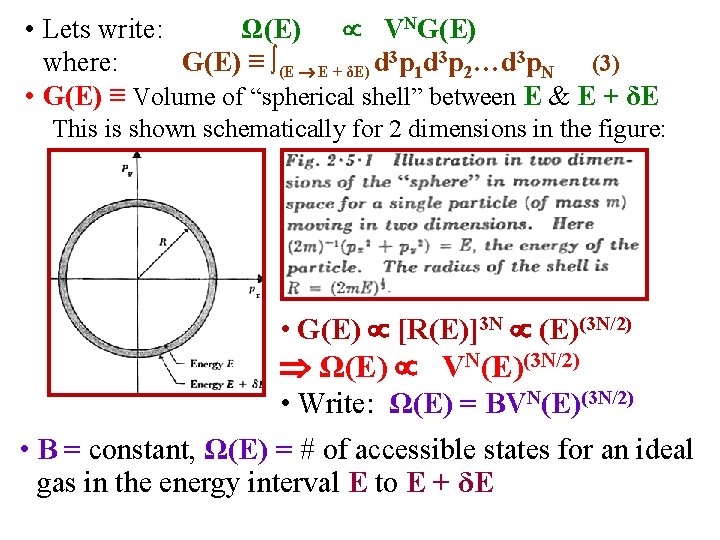
• Lets write: Ω(E) VNG(E) where: G(E) ≡ ∫(E E + δE) d 3 p 1 d 3 p 2…d 3 p. N (3) • G(E) ≡ Volume of “spherical shell” between E & E + δE This is shown schematically for 2 dimensions in the figure: • G(E) [R(E)]3 N (E)(3 N/2) Ω(E) VN(E)(3 N/2) • Write: Ω(E) = BVN(E)(3 N/2) • B = constant, Ω(E) = # of accessible states for an ideal gas in the energy interval E to E + δE
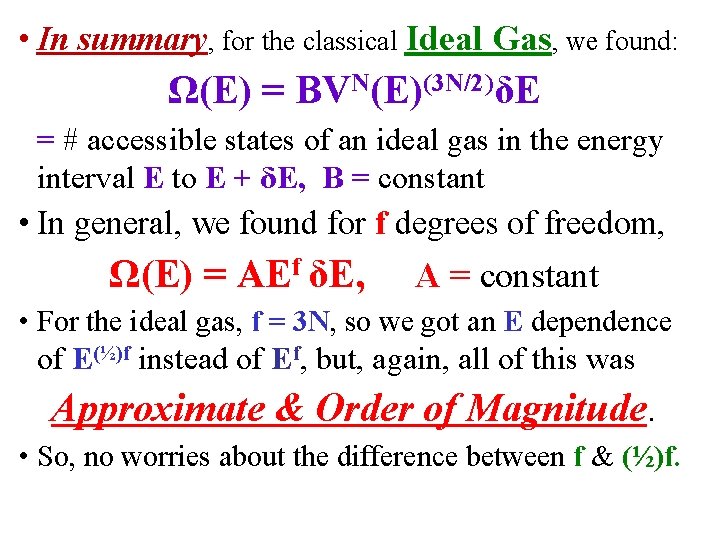
• In summary, for the classical Ideal Gas, we found: Ω(E) = BVN(E)(3 N/2)δE = # accessible states of an ideal gas in the energy interval E to E + δE, B = constant • In general, we found for f degrees of freedom, Ω(E) = AEf δE, A = constant • For the ideal gas, f = 3 N, so we got an E dependence of E(½)f instead of Ef, but, again, all of this was Approximate & Order of Magnitude. • So, no worries about the difference between f & (½)f.
- Slides: 18