Priority Setting in Universal Health Coverage The role















- Slides: 21

Priority Setting in Universal Health Coverage The role of Health Intervention & Technology Assessment Dr. Kees de Joncheere Director EMP department EMP Technical Briefing Seminar, November 2014 1| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Outline • Concept of UHC and Priority Setting • What is Health Intervention and Technology Assessment • Ongoing programmes of work contributing to HITA in WHO • HTA for medicines evaluation • The way forward 2| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Universal health coverage (UHC) The goal of universal health coverage is to ensure that all people obtain the health services they need without suffering financial hardship when paying for them. – A strong, efficient, well-run health system that meets priority health needs – Affordability – a financing system to avoid financial hardship – Access to essential medicines and other health technologies – Sufficient capacity of well-trained, motivated health workers to provide the services needed l Resources are scarce in all settings and forms of priority setting are inevitable 3| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

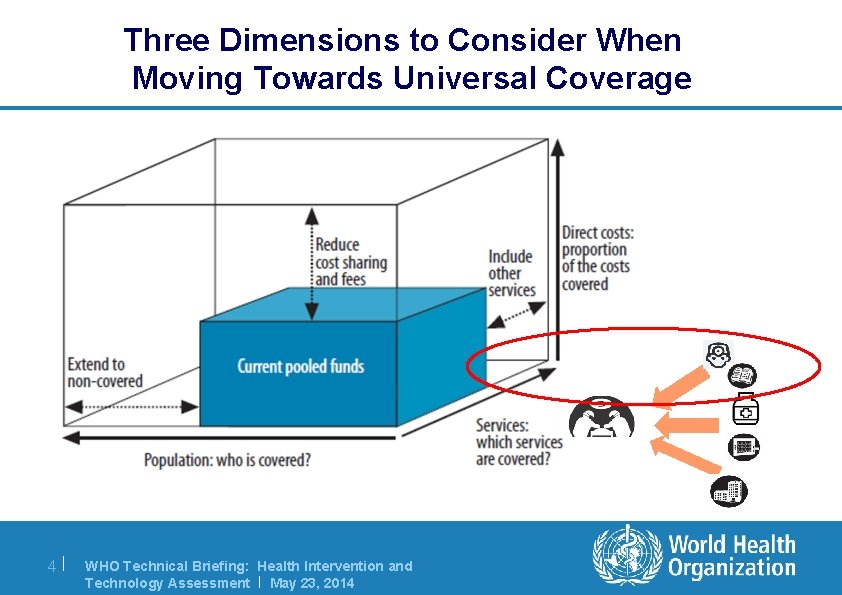
Three Dimensions to Consider When Moving Towards Universal Coverage 4| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Considerations in priority setting l In moving towards UHC, questions focus on: – The population covered by the package of interventions: Who ? – The services that can be provided: Which services ? – The proportion of service costs that can be covered: How much ? l Health technology assessment (HITA) is an important process to aid priority-setting within the services axis of the UHC cube 5| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

What is Health Intervention & Technology Assessment? l Health technology is the application of organized knowledge and skills in the form of interventions, devices, medicines, vaccines, procedures and systems developed to solve a health problem and improve quality of lives l Technology assessment in health care is a multidisciplinary field of policy analysis. It studies the medical, social, ethical, and economic implications of development, diffusion, and use of health technology. l HITA does not make the decisions, however the systematic assessment of the evidence makes the trade-offs between alternative actions clear 6| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

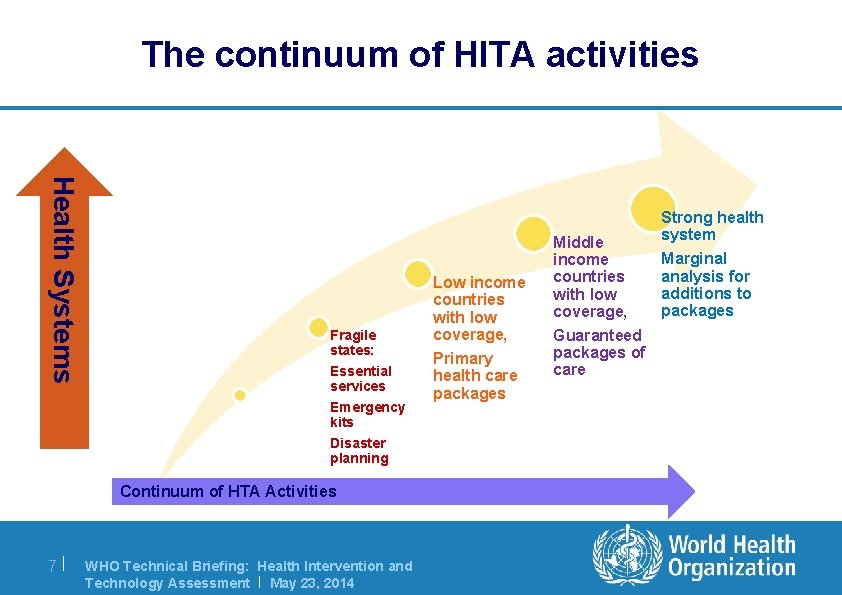
The continuum of HITA activities Health Systems Fragile states: Essential services Emergency kits Disaster planning Continuum of HTA Activities 7| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014 Low income countries with low coverage, Primary health care packages Middle income countries with low coverage, Guaranteed packages of care Strong health system Marginal analysis for additions to packages

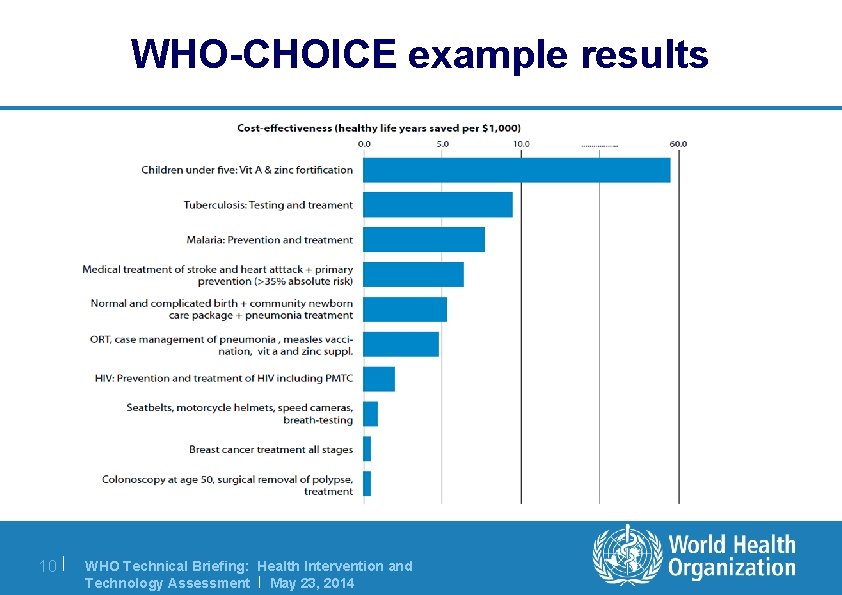
Uses of HITA methods in WHO l WHO Model List of Essential Medicines l Package of Essential Noncommunicable (PEN) disease interventions for primary health care, 'best buys' for NCDs l WHO-CHOICE, CHOosing Interventions that are Cost Effective global database of around 500 health technologies l One. Health Tool designed to inform national strategic health planning in lowand middle-income countries. l Assessing medical devices and assistive devices for an ageing population 8| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Examples of ongoing HITA in WHO l WHO Model List of Essential Medicines – First published in 1977 – Updated every 2 years – 156 countries have essential medicines lists l One. Health Tool for Costing and Strategic planning – Development began in 2008 – Released in 2012 – Has to date been used in over 25 countries l WHO-CHOICE – Ongoing since 1998 – Development of cost-effectiveness analyses of interventions covering all WHO regions 9| WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

WHO-CHOICE example results 10 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

What is needed for HITA? l Assessment is based on collecting, evaluating, and systematically reviewing all available evidence for the intervention or technology being considered – Types of data include epidemiological, economic, health impact, expert opinion – Methods include assessing the quality of available information, systematic review and meta-analysis, surveys, feasibility, affordability and ethical considerations l Multidisciplinary skills are needed to assemble and interpret the data l Countries with the greatest need often have the least capacity l Different types of HITA vary in scope, time and resources required – Full scale health technology assessment report – Contextualization of reports produced by others 11 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Evaluation of new medicines l For market entry : – quality, efficacy, safety ; benefit/risk assessment l For reimbursement – Medical need and severity of disease – Health gain and added therapeutic value – Availability of alternatives – Cost-effectiveness – Budget impact – Equity considerations –… 12 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Evaluation of new medicines l For medical practice – Evaluation by drug bulletins, professional associations on the “place in therapy” of a new medicine – Consistency between reimbursement indications and therapeutic guidelines ? – Reviews by national HTA bodies 13 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Implications of cost-effectiveness analysis for reimbursement l Health-based reason to justify a price premium for the proposed drug l Relate extent and nature of health gain to justify price increase, including cost off-sets in health sector l Common outcome measure (QALY, life year gained, . . ) l Pristine value judgement 14 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Economic evaluations l Two main approaches – on a “cost-minimisation” basis – as “acceptably cost-effective” l Two main “levers” – restrict to particular patients – price of the proposed drug 15 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Reimbursement decision-making process : after the initial reimbursement decision … l Post-listing reviews (at least annually) – prices – restrictions and listings l Post-listing monitoring (at least annually) – usage (including predicted versus actual) – cost to reimbursement system l Coordinate post-listing activities 16 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

International collaboration on evaluation of medicines l Health care systems are different l Issues tend to be the same : costs drivers and evidence l Basis for common guidance, and exchange of information, and “lessons learned” ? ! – – – – 17 | MEDEV EU network of Pricing and Reimbursement authorities EUnet. HTA INAHTA, ISPOR, HTAi Hi. TAP and South East Asia network REDETSA Latin America network PPRI and PHIS networks WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Making cost-effectiveness evaluations work (1) ( do countries have the resources to do this l Separate licensing and reimbursement decisions l Positive list l Price negotiations l Ability to restrict indications l Adequate guidelines for submissions l Competent evaluations of submissions l Consistent and informed decision-maker 18 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014 ? )

Making cost-effectiveness evaluations work : decisionmaking process (2) l Inclusiveness in decision-making and input from all stakeholders l Reimbursement committees with external experts : managing potential conflict-of-interests l Health Technology Assessment agencies and committees often not directly linked with reimbursement decisions 19 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

Use of cost-effectiveness analysis in reimbursement l Growing requirements and increasing need for resources l “Silo-budgeting” limits application l Shift to “risk-sharing” with patient registries : from paying for the medicine to buying an agreed upon therapeutic outcome l CEA needs to relate to goals, values, and priorities of the health care systems l RCTs do not provide all information needed : discuss with industry the need for additional trials l How to deal with “point-decisions” vis-à-vis re-assessment of the evidence ? l Discussions on appropriateness of QALY`s l Discussions on thresholds 20 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014

The way forward l WHO HQ plans to undertake a global mapping survey of current capacity and perceived needs for HITA in member states l A focus of moving forward should be on – Advocacy and promotion of priority setting (including HITA) best practices – Facilitate sharing of technology assessment and experiences among countries including through the development of platforms for information exchange – Capacity building activities including networks where appropriate 21 | WHO Technical Briefing: Health Intervention and Technology Assessment | May 23, 2014