Prior Thoughts Knowledge Have I ever had any











































































































- Slides: 114

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

Classroom Chemistry Grade 5 Science Mr. Pukalo

Introduction Mr. Pukalo l What is important to me? l Following God, family, friends l What do I like to do in my spare time? outdoors, sports, reading, music l l My favorite video in the world http: //www. youtube. com/watch? v=3 Ya 12 I 0 36 lg&safety_mode=true&persist_safety_m

In the Classroom What is important? l Fun (but this can only happen if…) l l Respect AND LISTENING to each others ideas/opinions (HANDS UP if you want to talk) No negative l Discussion vs. writing notes l Share and value each others ideas l

Your turn Does the class like videos? ? l 1. What is important to you for a teacher to do? l 2. How can we create a class that will be fun but ensure we are learning what we have to learn? l 3. What is your responsibility to ensure this will happen? l Share experiments/ideas l

Check it out! Intro video l http: //www. youtube. com/watch? v=RFe 5 kr Faq. UI&safety_mode=true&persist_safety_ mode=1 l

What is Chemistry? What do you know about Chemistry? (KWL) l What do you like? l What do you dislike? l What do you hope to learn? l

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

What is Chemistry The study of Matter and how it interacts l Solids, liquids & gas l

Beginning of Chemistry l l l In the Beginning (1: 1 -2) 1 In the beginning God acreated the bheavens and the cearth. 2 The earth was formless and empty, and darkness covered the deep waters. And the d. Spirit of God was hovering over the surface of the waters. http: //www. youtube. com/watch? v=w. JYa. Abr. Wsv. A&featur e=related&safety_mode=true&persist_safety_mode=1 Piper the glory of God http: //www. youtube. com/watch? v=Ibc 8 s. D 5 sgw&feature=related&safety_mode=true&persist_safety_m ode=1 Fingerprint of God

Classroom Chemistry l What do Chemists do? l http: //www. youtube. com/watch? v=66 kuh. Jk QCVM

Final Thoughts What did I learn today? l Favorite part of today’s lesson? l

Other Careers Using Chemistry Doctor l Engineer l Pharmacist l Chemistry Teacher l

Where does Chemistry take place? l In the natural world l In the manufactured world l Daily life

Social Implications The field of chemistry shapes society and is shaped by society (both effect each other) l What are the possible social implications in chemistry? l http: //www. youtube. com/watch? v=RFe 5 kr. Fa q. UI&safety_mode=true&persist_safety_m ode=1

Social Implications Communication devices, media, ipods l Employs millions of people l Healthier foods l Edible paint l Rice that can grow anywhere l Health and medical (drugs) l Ice, gum, l

In your everyday life where do you see Chemistry? l http: //www. youtube. com/watch? v=Q 3 Tt 2 EM 4 e. U&safety_mode=true&persist_safety_mode=1

How is chemistry shaped by society l Meeting the needs and wants of society

How is society shaped by chemistry? l What changes/technology advancements have been made by technology?

Final Thoughts l 4. Where does Chemistry take place? l 5. Social implications

Your Turn 1. What should I keep doing ? (Favorite part of the lessons so far. ) l 2. What should I stop doing? l 3. What should I start doing? l 4. Favorite or most interesting part. l 5. Least favorite or interesting part so far. l

Unit Road Map Students will: 1. Recognize and identify examples of mixtures. 2. Apply and evaluate a variety of techniques for separating different materials. 3. Distinguish substances that will dissolve in a liquid from those that will not, and demonstrate a way of recovering a material from solution. 4. Demonstrate a procedure for making a crystal. 5. Recognize that the surface of water has distinctive properties, and describe the interaction of water with other liquids and solids. l 6. Produce carbon dioxide gas through the interaction of solids and liquids, and demonstrate that it is different from air. 7. Distinguish reversible from irreversible changes of materials, and give examples of each. 8. Recognize and describe evidence of a chemical reaction. Explain how the products of a reaction differ from the original substances. 9. Use an indicator to identify a solution as being acidic or basic.

States of Matter

Check it out! Music Video l http: //www. youtube. com/watch? v=t. BQcp. F _j 5 Xg&feature=related l

Prior Thoughts & Knowledge Solids, Liquids and Gases l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

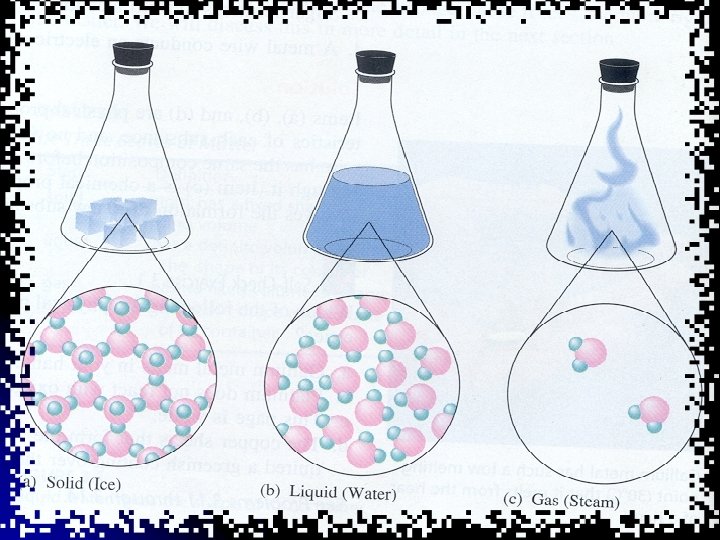
Matter l Matter is the substance of which physical objects are composed. Matter is anything that takes up space. l It can be solid, liquid or gas. l

Brain Pop – What makes a state of matter? l l l What is the difference between solids, liquids, and gases? What is a phase change? What exactly is plasma? What state of matter is oobleck? What is aerogel? Are there seven states of matter? Is a bucket of snow heavier than a bucket of water? Why is ice so cold? What is Boyle's Law? Why can I see my breath on a cold day? http: //www. brainpop. com/science/matterandchemistry/statesofmatte r/

States of Matter Review

Solids – MOST DENSE l Molecules are attached and bunched together in a “solid” form. l Doesn’t change shape easily. l Another solid cannot pass through easily Take up least space l

Examples

Liquid l Molecules fill the space of the container they are in. l They can shape easily. l A solid can pass through it.

Examples

Gas – Least Dense l Molecules freely move around. They are not closely bound together. l Changes shape easily l A solid can pass through it easily. Gases Video http: //www. youtube. com/ watch? v=Wc. NDYe 6 Ka. Tk Take up most space l l

Examples


Review questions What is matter? is the substance of which physical objects are composed. l Liquid l Fixed volume but no fixed shape l Gas (add energy) l No fixed volume and no fixed shape l Solids (lower temperature remove energy) l Fixed volume and fixed shape l

Brain Pop – Measuring Matter l l l What is matter? What is the relationship between mass, weight, and gravity? I want to know how something can have the same volume yet a different mass? What is displacement and how do we use it? What’s the difference between mass and weight? My school is having a contest to guess how many M&Ms are in a jar. How do you figure it out? What is Boyle's Law? What is volume? What is a liter? What are measuring cups? http: //www. brainpop. com/science/matterandchemistry/measuringma tter/

Bill Nye music video http: //www. youtube. com/watch? v=Uv. Rv. O YCj. UP 0&feature=related l Computer Simulation http: //www. bbc. co. uk/schools/ks 2 bitesize/s cience/materials/changing_state/play. shtm l l

Review of Matter 1. What are three states of matter? l 2. What is the major differences between the three states of matter: l Shape l Volume l

Final Thoughts What did I learn today? l Favorite part of today’s lesson? l

Quiz Time 1. Put first and last name on test l 2. Circle the correct answer l 3. Absolute silence (talk = 0) l 4. Put your hand up if you have a question l

Marking 1. Marked by “Sarah” l 2. Questions please ask l

Review States of matter l Solid, liquid and gas are called the three states of matter. l Materials can be changed from one state to another by heating or cooling. l

Review Does air have mass? l Are different substances solid, liquid and gas at different temperatures? l If you want to melt or boil something what would you do? l If you wanted to create a vehicle what substance would you want to use? l

Homework Diet coke (7 people) MINT mentos Think about find or do a cool experiment write it down, draw a picture and show the class on Monday.

Quiz feedback Put first & last name on all tests l Use a different color to mark l Great job!! Keep up the good work. l

Changing States of Matter

Review 3 States of matter l Matter is Liquid l Fixed volume but no fixed shape l Gas (add energy) l No fixed volume and no fixed shape l Solids (lower temperature remove energy) l Fixed volume and fixed shape l

Check it out! l http: //www. youtube. com/watch? v=5 b. CNdd IG 7 CI&feature=fvw&safety_mode=true&pe rsist_safety_mode=1

Prior Thoughts & Knowledge How can we change the state of matter? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

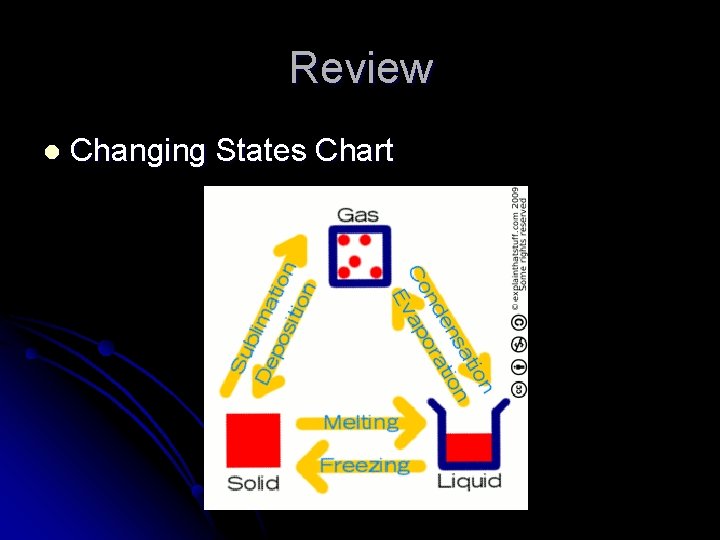
Prior Thoughts & Knowledge Liquid to Solidl Freezing l Solid to Liquid – l Melting l Liquid to Gasl Evaporation - Gas to Liquid- Condensation l l song http: //www. youtube. com/watch? v=Y 9 g 0 jrvd-

l l l Heating If ice (solid) is heated, it changes to water (liquid). This change is called melting. If water (liquid) is heated, it changes to water vapour (gas). This change is called evaporation. Cooling If water vapour (gas) is cooled, it changes to water (liquid). This change is called condensing. If water (liquid) is cooled, it changes to ice (solid). This change is called freezing.

Review l Changing States Chart

l l l The water cycle Water on the earth is constantly moving. It is recycled over and over again. This recycling process is called the water cycle. a. Water evaporates into the air The sun heats up water on land, and in rivers, lakes and seas and turns it into water vapour. The water vapour rises into the air. b. Water vapour condenses into clouds Water vapour in the air cools down and changes back into tiny drops of liquid water, forming clouds. c. Water falls as rain The clouds get heavy and water falls back to the earth in the form of rain or snow. d. Water returns to the sea Rain water runs over the land collects in lakes or rivers, which take it back to the sea. The cycle starts all over again.

Brain Pop Changing States l Questions l What is a phase change? How is the melting point of one object the freezing point of another? What is the difference between solids, liquids, and gases? What are chemical and physical changes? Sublimation sounds neat. Can you tell me about it? Why does water expand when it freezes? ¿Qué es un Géiser? Why is ice so cold? Why does ice melt? What is supercooling? http: //www. brainpop. com/science/matterandchemistry/matterchangi ngstates/ l l l l l

Changing States Review l What causes change of states? l Temperature and pressure (physical change) Melting (solid liquid) Heat of fusion – breaking ice bonds l l

Brain Pop Changing States l Questions l What is a phase change? How is the melting point of one object the freezing point of another? What is the difference between solids, liquids, and gases? What are chemical and physical changes? Sublimation sounds neat. Can you tell me about it? Why does water expand when it freezes? ¿Qué es un Géiser? Why is ice so cold? Why does ice melt? What is supercooling? http: //www. brainpop. com/science/matterandchemistry/matterchangi ngstates/ l l l l l

Mixtures

Mixtures l l Matter can generally be mixed with other types of matter. A mixture is a physical combination of two or more pure substances (elements or compounds) A mixture is when particles of one substance mixes with particles of another substance. They are generally pure substances. Materials in a mixture can be separated using techniques such as filtration, screening and sedimentation

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

Where do we see mixtures? l Examples: recipes, construction-concrete, water, lemonade, salad dressings.

Separating Mixtures l How can you separate substances from a mixture? l Methods of Separating: sieves, magnets, air, water, evaporation, distilling, filtering

Methods of Separating l Sieves: can be used to separate solids. l Magnets: can pick magnetic objects, from non-magnetic.

Methods of Separating l Air: you can blow away lighter substances, to leave heavier ones. l Water: some substances will float or sink based on their buoyancy.

Methods of Separating l Evaporation: evaporate the liquid and leave the solid. l Distilling: the processing of vaporizing into gas and then condensing back into a liquid

Methods of Separating Filtration: using a filter and pouring the liquid through to separate the solid. l http: //www. bbc. co. uk/schools/scienceclips/ ages/10_11/rev_irrev_changes. shtml l http: //www. mcgrawhill. ca/school/applets/bcs cience 7/mixtures/index. htm MASTER # 2/ Seperating mixtures observation booklet

Brainpop - Mixtures l l l http: //www. brainpop. com/science/matterandchemistry/compoundsan dmixtures/ What’s the difference between a mixture and a compound? What is a colloid? Does the type of bonding affect the physical properties of a compound? Do compounds and mixtures cause chemical or physical changes? What is a solution? Can you separate mixtures? Why does sodium explode if it touches water? Why doesn’t that happen with the sodium in salt? What does “heterogeneous” mean? What are catalysts? What is homogenization?

Review Compound = chemical change, properties CHANGE mixture = physical change, retains original properties l Hetrogeneous mixture – can see two substances l Homogenous mixture – LOOKS LIKE sz m. ONE SUBSTANCE! l

Review l What is the difference between a homogenous mixture and a compound? l THEY LOOK THE SAME!!

Mixtures l Homogeneous l Hetrogeneous l http: //www. youtube. com/watch? v=MNTIRa rrvo. U&feature=related&safety_mode=true &persist_safety_mode=1

Final Thoughts What did I learn today? l Favorite part of today’s lesson? l

Mixing Liquids & Gases

Check it out! l http: //www. youtube. com/watch? v=B 3 kode Qn. Qv. U&feature=channel&safety_mode=tr ue&persist_safety_mode=1

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

Mixing Gas - Carbon Dioxide Air is composed of 78% Nitrogen, 21% oxygen and 1% other gases like carbon dioxide, water vapour, helium, etc. l We breath oxygen. l Carbon Dioxide is the gas we breath out. That is formed from burning fuel. l Carbon dioxide is heavier than oxygen l Gas in a Bag activity. BLM #12 l

Mixing Liquids Some liquids mix completely and are unable to be separated. eg: Milk and Tea l Some liquids do not dissolve in others and are more buoyant. eg: oil and water l Some liquids are heavier, less buoyant and settle on the bottom. eg: syrup l

Mixing Liquids l Some liquids react to each other. Eg: vinegar and milk. l Some liquids are able to dissolve solids, while some are not. l Lemonade is an example of a liquid mixture.

Activity: Layering Liquids l Why were the liquids able to be layered and not mixed? l Try mixing two different liquids, record your observations.

Lifesaver Experiment l BLM #6 and 7 l Observe how long the lifesaver takes to dissolve l The lifesaver dissolved into the water l Dissolve is when a solid crumbles into a liquid. l Can you make it dissolve faster?

Lifesaver Experiment: Inferences Manipulated Variablel Responding Variable- amount of time it will take to dissolve a lifesaver. l

Solutions l A homogeneous mixture in which the solute is uniformly distributed throughout the solvent. l Solute- The substance that is being dissolved in a solution. l Solvent- the substance that does the dissolving in a solution

Suspension (Master #8) l A mixture in which very small particles of a solid remain suspended without dissolving. l Heterogeneous Mixture- when one substance is unevenly mixed with another.

Separating Solutions Filtering l Pouring off the liquid l Evaporation l l Solution to Recovery Activity

Crystal Experiment

Brain Pop - Crystals l l l l l What are crystals and how do they form? What is a lattice? How can you grow your own crystals? What are some different types of crystals? Why do different crystals have different colors and shapes? What are the seven main shapes of crystals? What is quartz? What is a geode? How can you tell if a crystal is really worth anything? What are sapphires?

Brain Pop - Liquid Crystals l http: //www. brainpop. com/science/earthsys tem/crystals/fyi/

Crystals (Master #10) l We can recover a dissolved substance by evaporation. l We can create crystals when the liquid evaporates.

Surface Tension

Check it out! l http: //www. youtube. com/watch? v=yied 0 a 0 DY 9 Q&safety_mode=true&persist_safety_ mode=1

Surface Tension l Water droplets are round and shaped like balloons l The film that forms on the surface of the water is called surface tension. l Surface tension is due to cohesion. An attraction of the molecules in water.

Surface Tension l Water is very cohesive. The water molecules act like glue. l Penny Challenge Paper Clip MASTER #11 l Why was the water able to bulge up? Surface tension-cohesion of water molecules.

Physical & Chemical Changes

Check it out! l Fireworks http: //www. youtube. com/watch? v=0 Mbf 1 Pf l_2 U&safety_mode=true&persist_safety_ mode=1

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

Brain Pop - Changes l http: //www. brainpop. com/science/matterandchemistry/pr opertychanges/ l What are chemical and physical changes? What is the difference between a reversible and a non-reversible change? Do compounds and mixtures cause chemical or physical changes? Why and how do apples turn brown? Is this a chemical or physical change? What is the difference between a physical change and a chemical change? When wood burns, is it a chemical or physical change? What are physical and chemical properties of matter? What is a phase change? Does the type of bonding affect the physical properties of a compound? What is rust? l l l l l

Chemical Reaction These are changes where two substances react chemically and they make a new substance. l a chemical reaction is a process by which one or more substances are transformed into one or more new substances l l Testing Powders Activity

Evidence of a Chemical Reaction an external indicator may accompany a reaction l colour change, l production of a gas l release heat l ‘formation of a precipitate l odour l

Reversible and Irreversible Changes l Reversible (physical) changes can go back to their original state. Irreversible (chemical) changes cannot go back to their original state. l Bill Nye Chemical Changes http: //www. youtube. com/watch? v=66 kuh. Jk QCVM l

(Chemical) Irreversible or Reversible (Physical) Heat l Light l Smell l Color change l Precipitate forms l

Chemical & Physical Changes l A chemical change is a change in matter in which one or more new substances is produced. This process is not easy to reverse. Some clues that a chemical change has taken place are a change in color, the release of gas, heat, light, or an odor. When you burn wood, the wood is chemically changed (into ashes) by the fire. A physical change is a change in matter in which no new substance is produced. This is usually a change from one state to another, and can be reversed. A good example of a physical change is water turning into ice and melting back into the liquid form again.

Mixture & Compound Review l A compound is a substance that is formed as the result of chemical changes. A mixture is a substance that results from physical changes. When atoms of two or more elements bond to form a new molecule, you’ve got a compound. Water is a compound, because it is made up of one hydrogen atom and two oxygen atoms. A mixture is when two or more elements or compounds are mixed together, but there is no chemical change. Lemonade is an example of a mixture—it mixes the elements that make up sugar and water and lemon juice, but these elements don’t form new molecules when they’re mixed together.

Irreversible Changes Online powerpoint l http: //www. slideshare. net/stanhopekris/rev ersible-and-irreversible-changes l Review reversible changes powerpoint l http: //www. bbc. co. uk/schools/ks 2 bitesize/s cience/materials/reversible_irreversible_ch anges/play. shtml l

Changes Summary activities and challenges http: //www. teachingandlearningresources. co. uk/6 d-science. shtml l Online Experiment (MASTER # 12) http: //www. bbc. co. uk/schools/scienceclips/a ges/10_11/rev_irrev_changes. shtml l

Acids & Bases

Check it out! l http: //www. youtube. com/watch? v=IIu 3 rr. Gf n. UU&safety_mode=true&persist_safety_m ode=1

Prior Thoughts & Knowledge Have I ever had any real life experience I can share? l What do I already know? l How did I learn this? l Is the source reliable and scientific? l How sure am I it is true? Absolutely sure, fairly confident or not sure? l

Brain Pop – Acids & Bases l l l http: //www. brainpop. com/science/matterandchemistry/acidsandbase s/ What are some examples of acids and bases that we use in our everyday lives? How come the acids in our tummy don’t burn us? Do acids burn your skin? What is p. H? What are organic bases? What is the Brønsted-Lowry theory? What is acid rain? Why do onions make us cry? Why does vinegar make eggs feel like rubber? What is the chemical formula for battery acid? Also, I know it’s dangerous as a liquid, but what about when it’s a powder?

Ph l l An acid is substance that has ph less than 7 A base is a substance that has a ph greater than 7. Neutral has a ph of 7 Both acids and bases are potentially harmful and they eat away at other substances.

Brain Pop - Ph l http: //www. brainpop. com/science/matterandchemistry/ph scale/ l What is p. H? I saw on a commercial that something was “p. H balanced. ” What does that mean? If I want to do my own tests at home to see what the p. H levels of things are, how do I get those little strips? What are some examples of acids and bases that we use in our everyday lives? What are organic bases? What is the Brønsted-Lowry theory? Where is the lemon on the p. H scale? What is the p. H of milk? l l l l

Acids and Bases l l Test household products online to see if acid or base. http: //www. proteacher. com/cgibin/outsidesite. cgi? external=http: //www. miamisci. org/ph/guide. html& original=http: //www. proteacher. com/110052. shtml&title=The p. H Factor

Indicators l l l An indicator changes colour in the presense of acidic and basic solutions. It will be one colour for acidic solutions, and another color for basic solutions. With some indicators, the intensity of the color increases with the intensity of the acidity of 'basic-ness' of the solution. Cabbage is a natural indicator. An Acid turns pink and a base turns green l l l l Examples of acid-base indicators: 1. ) Litmus Paper acidic solutions will turn the blue litmus paper to red while basic solutions will turn the red litmus paper to blue 2. ) Congo Red basic solutions will change the color of the entire solution into blue

Litmus Paper l Video http: //www. youtube. com/watch? v=z. TLi. JE-j 1 I&safety_mode=true&persist_safety_mode=1 l Litmus paper is used to determine if a liquid is acidic or basic Red Paper- acid stays red, base turns it blue l Blue Paper- acid turns it red, base stays blue. l Neutral- blue paper stays blue, red paper stays red. l

Final Thoughts What did I learn today? l Favorite part of today’s lesson? l

Nano Technology l http: //www. brainpop. com/science/matteran dchemistry/nanotechnology/