Principles of Spectrophotometer Medical Biochemistry and Molecular biology

Principles of Spectrophotometer Medical Biochemistry and Molecular biology Department
1. Recognize the principles of photometry and the related laws (Beer-Lambert’s laws). 2. Identify the main parts and the uses of the photometer. 3. Practice the steps of proper use of a photometer. 4. Determine the concentration biomolecule in a given sample. Extended Modular Program of certain 2
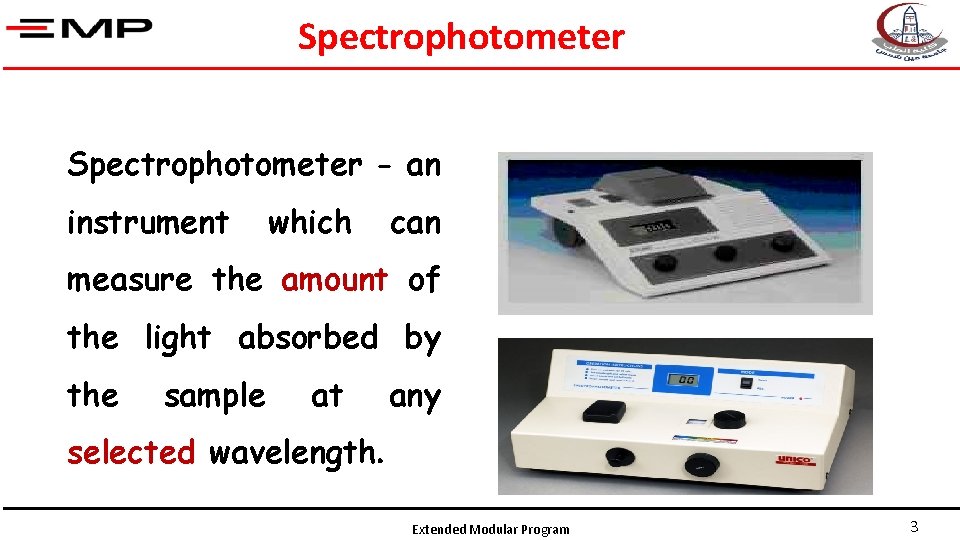
Spectrophotometer - an instrument which can measure the amount of the light absorbed by the sample at any selected wavelength. Extended Modular Program 3
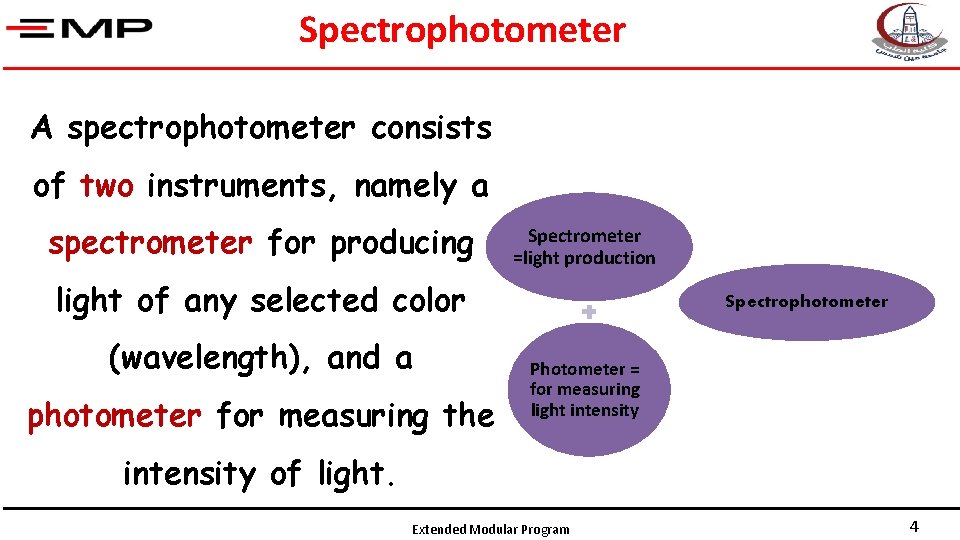
Spectrophotometer A spectrophotometer consists of two instruments, namely a spectrometer for producing Spectrometer =light production light of any selected color (wavelength), and a photometer for measuring the Spectrophotometer Photometer = for measuring light intensity of light. Extended Modular Program 4
Outlines 1. What is light? 2. Spetrophotometeric laws Absorption, Transmission 3. Spectrophotometer 1. Principle 2. Construction 3. Application Extended Modular Program 5

What is light? Light is a mixture of different electromagnetic wave lengths Extended Modular Program 6
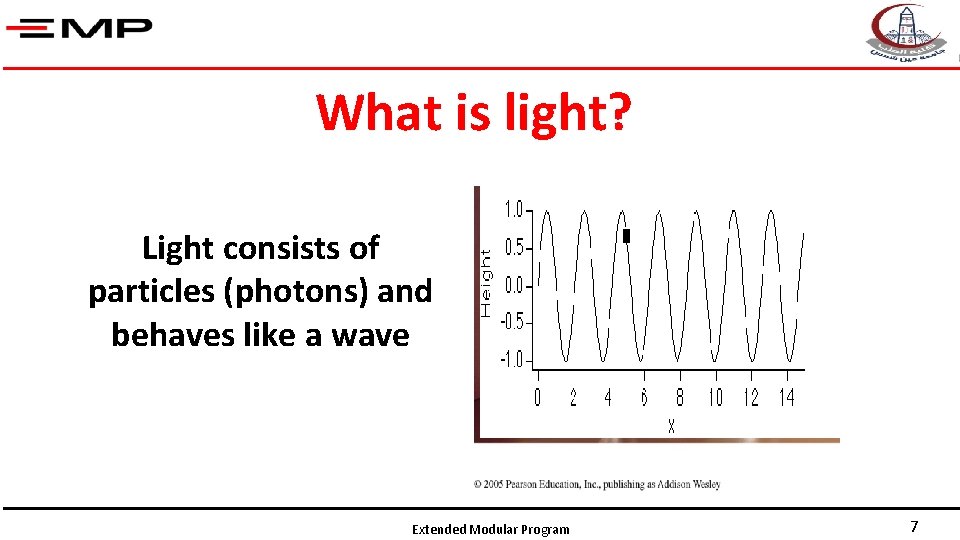
What is light? Light consists of particles (photons) and behaves like a wave Extended Modular Program 7
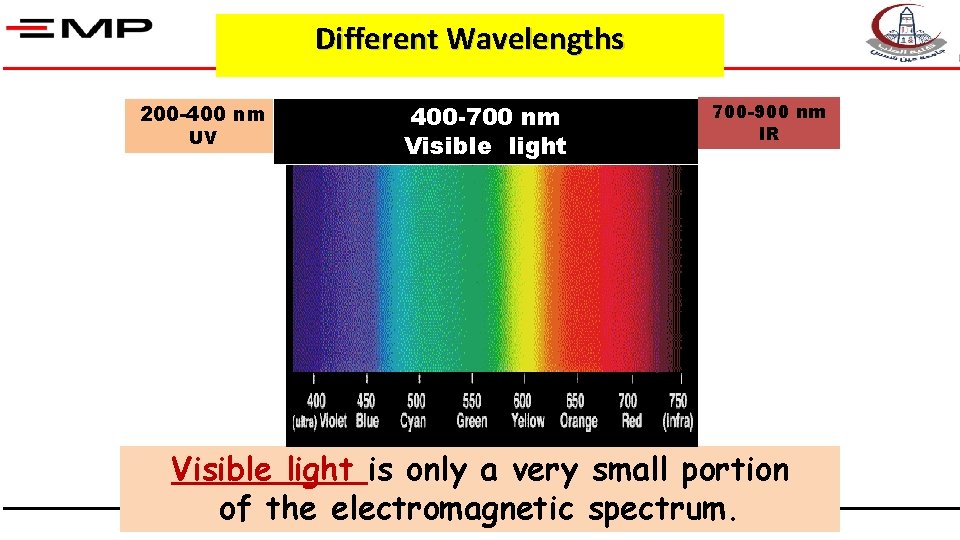
Different Wavelengths 200 -400 nm UV 400 -700 nm Visible light 700 -900 nm IR Visible light is only a very small portion of the electromagnetic spectrum.
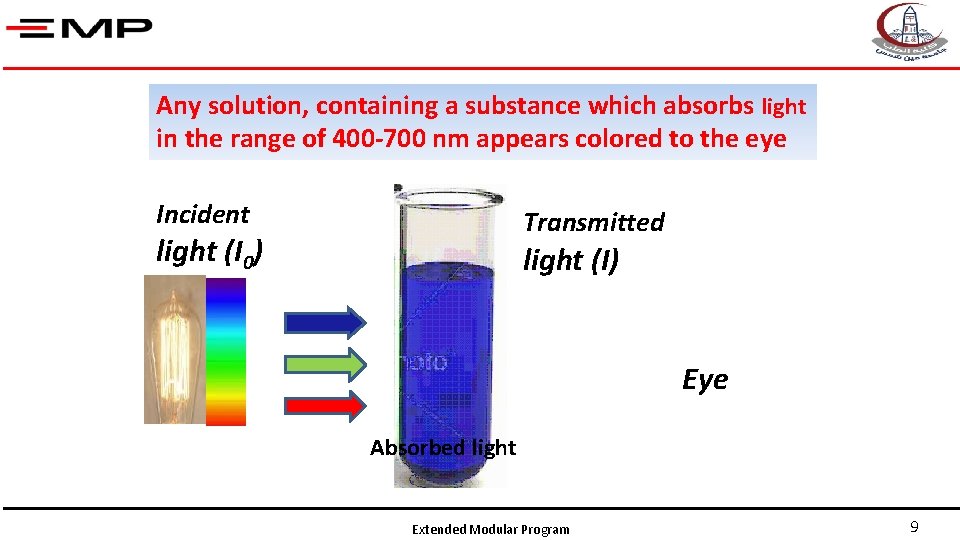
Any solution, containing a substance which absorbs light in the range of 400 -700 nm appears colored to the eye Incident Transmitted light (I 0) light (I) Eye Absorbed light Extended Modular Program 9
Incident Transmitted light (I 0) light (I) Absorbed light Extended Modular Program Eye 10
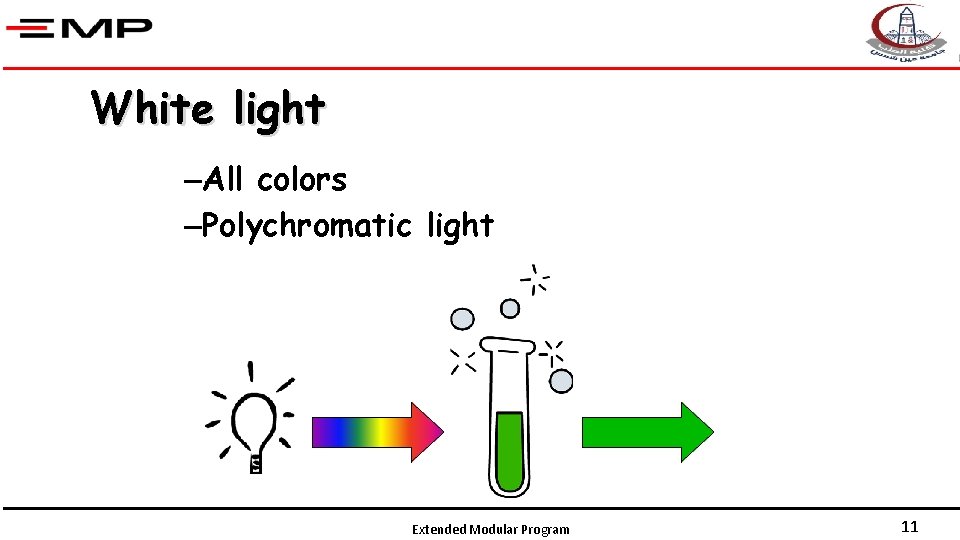
White light –All colors –Polychromatic light Extended Modular Program 11
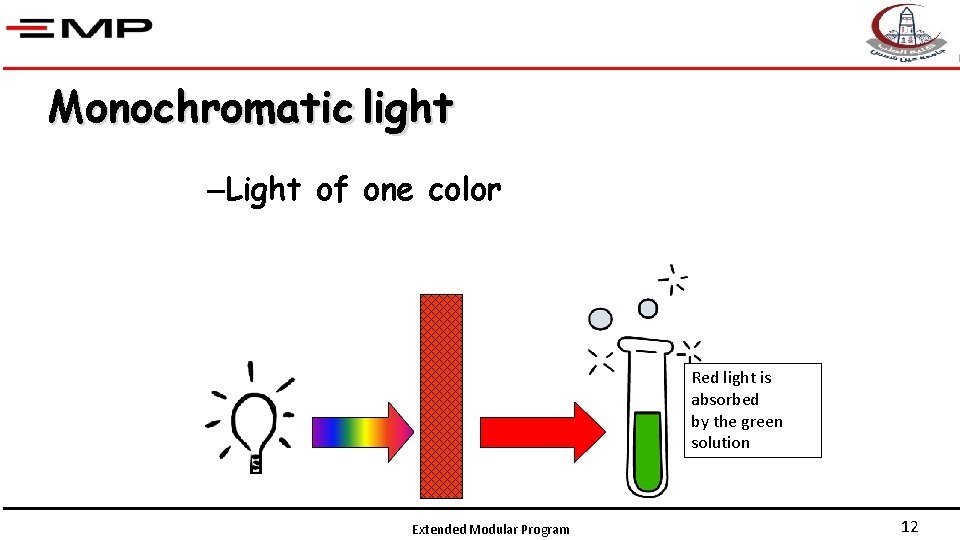
Monochromatic light –Light of one color Red light is absorbed by the green solution Extended Modular Program 12

Why does Color appear “deeper” with more Concentration? More molecules means more light is “absorbed” Extended Modular Program 13
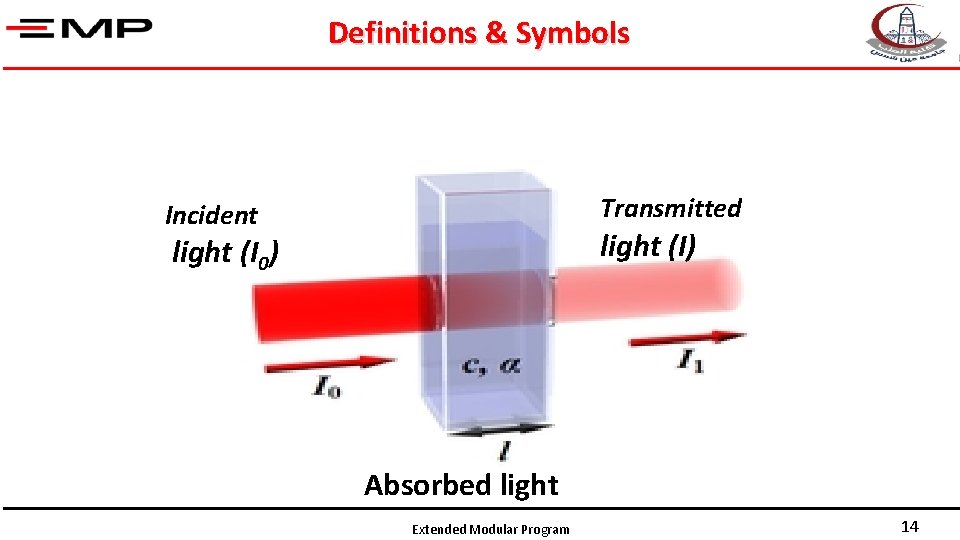
Definitions & Symbols Transmitted Incident light (I) light (I 0) Absorbed light Extended Modular Program 14
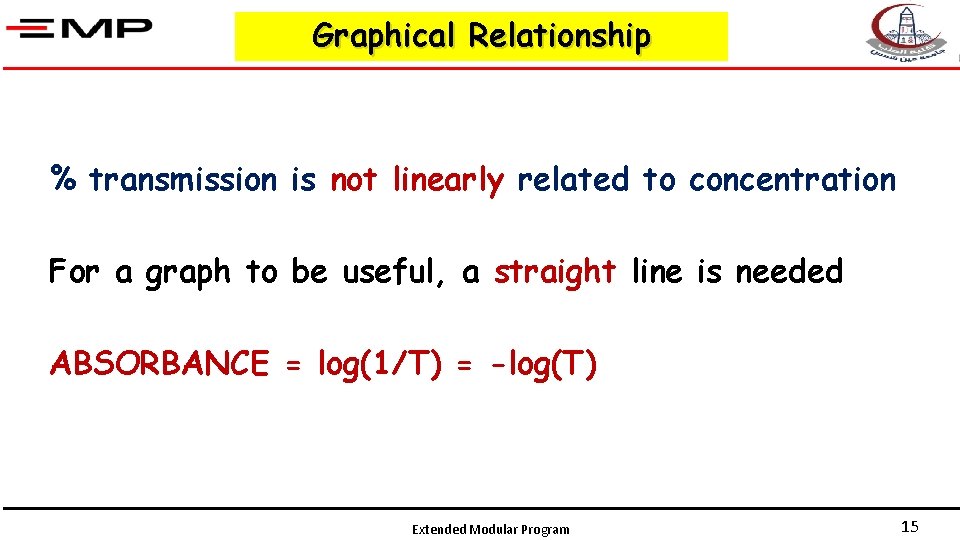
Graphical Relationship % transmission is not linearly related to concentration For a graph to be useful, a straight line is needed ABSORBANCE = log(1/T) = -log(T) Extended Modular Program 15
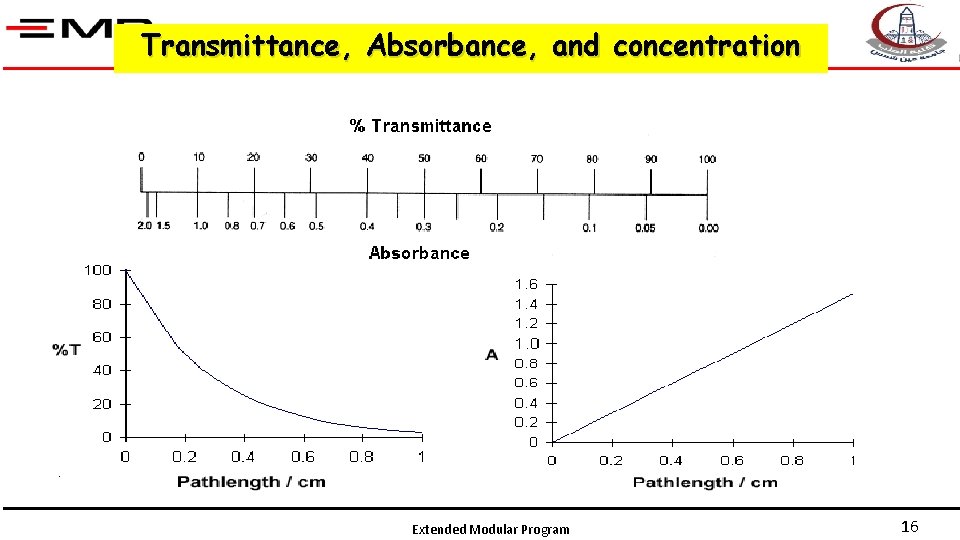
Transmittance, Absorbance, and concentration Extended Modular Program 16
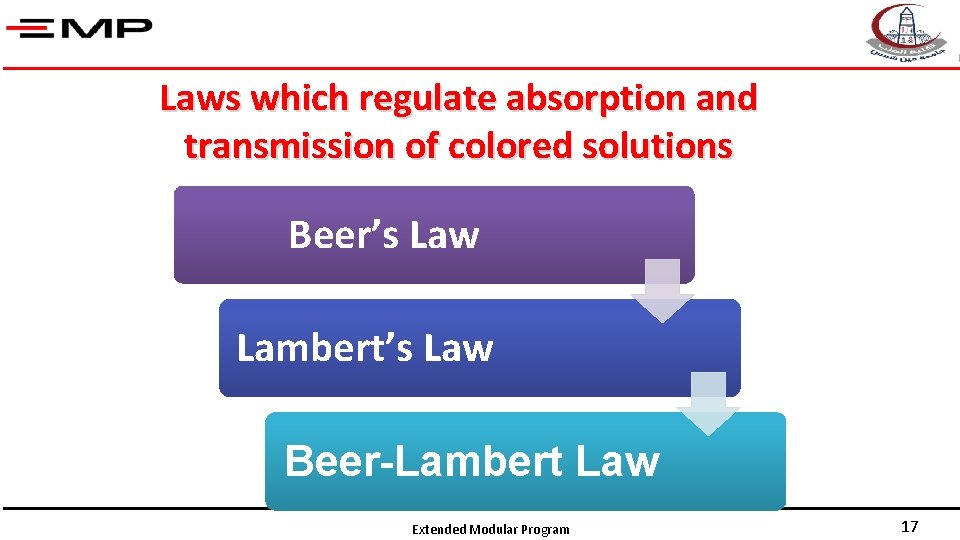
Laws which regulate absorption and transmission of colored solutions Beer’s Law Lambert’s Law Beer-Lambert Law Extended Modular Program 17

Beer’s Law identifies the relation between color and concentration AαC Beer’s Law states that the absorbance of light by a solution is directly proportional to its concentration Extended Modular Program 18
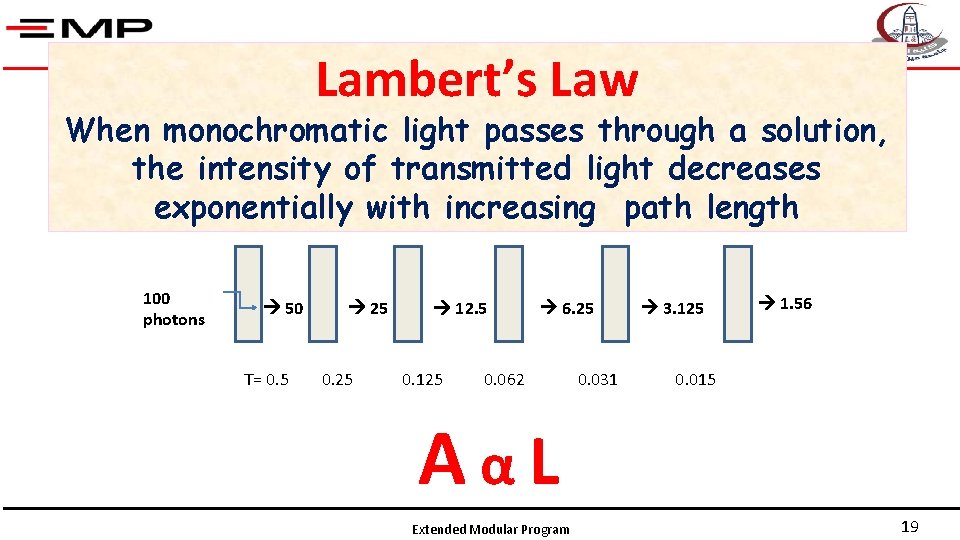
Lambert’s Law When monochromatic light passes through a solution, the intensity of transmitted light decreases exponentially with increasing path length 100 photons 50 T= 0. 5 25 0. 25 12. 5 0. 125 6. 25 0. 062 0. 031 3. 125 1. 56 0. 015 AαL Extended Modular Program 19
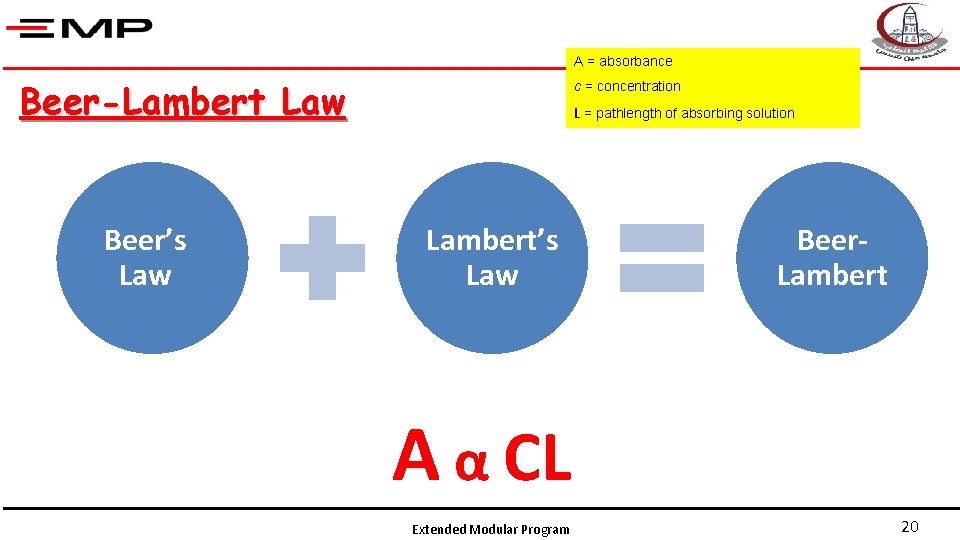
A = absorbance Beer-Lambert Law Beer’s Law c = concentration L = pathlength of absorbing solution Lambert’s Law Beer. Lambert A α CL Extended Modular Program 20
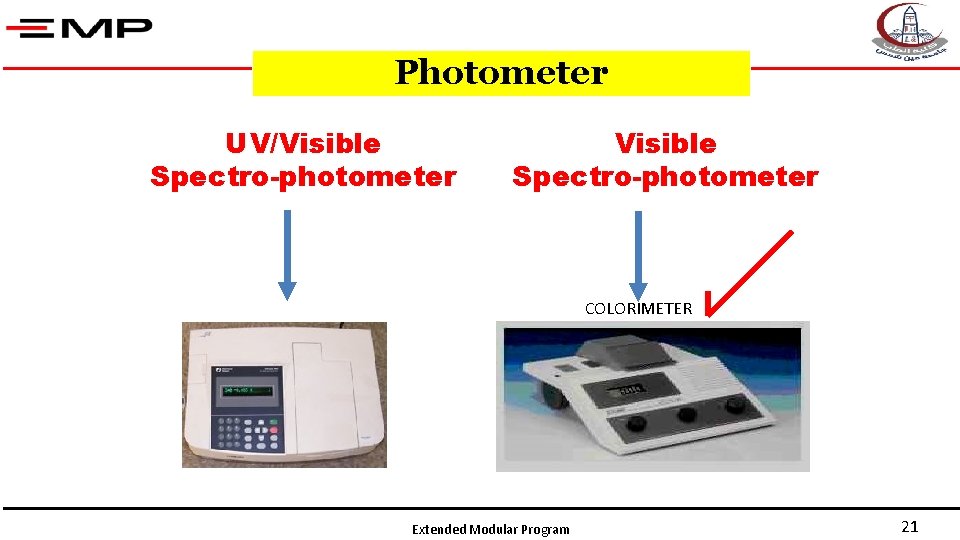
Photometer UV/Visible Spectro-photometer COLORIMETER Extended Modular Program 21
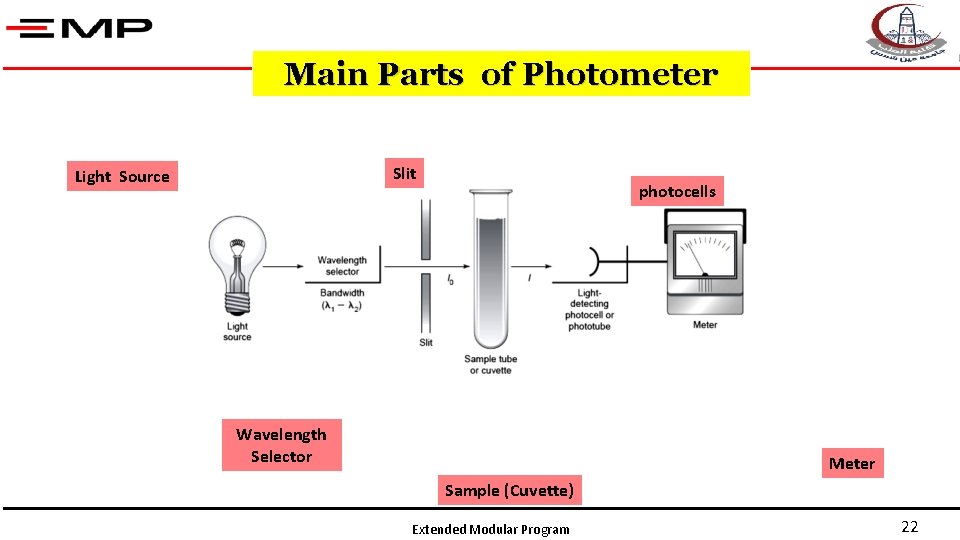
Main Parts of Photometer Slit Light Source photocells Wavelength Selector Meter Sample (Cuvette) Extended Modular Program 22
1 - Light Sources Visible Spectrophotometer Tungsten Lamp Extended Modular Program 23
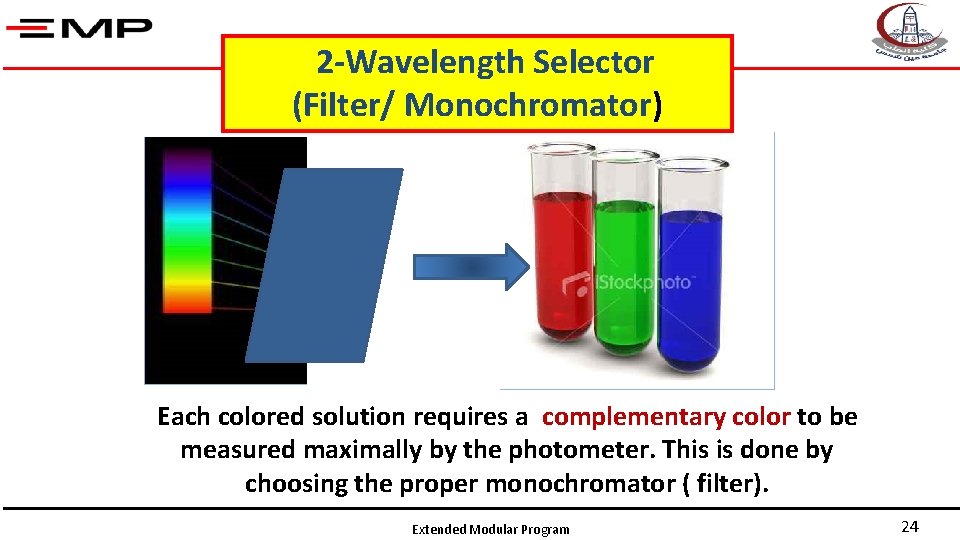
2 -Wavelength Selector (Filter/ Monochromator) Each colored solution requires a complementary color to be measured maximally by the photometer. This is done by choosing the proper monochromator ( filter). Extended Modular Program 24
3 - Slit It is necessary to adjust the intensity of the incident light (I◦) to form a Slit Extended Modular Program 25
4 - Sample cuvettes Visible Spectrophotometer Glass-plastic Extended Modular Program 26
5 - Light-Detecting photocells Spectrophotometers detect the transmitted light (I) Extended Modular Program 27
6 - Meter A = numerical scale with no unit or Digital Extended Modular Program 28
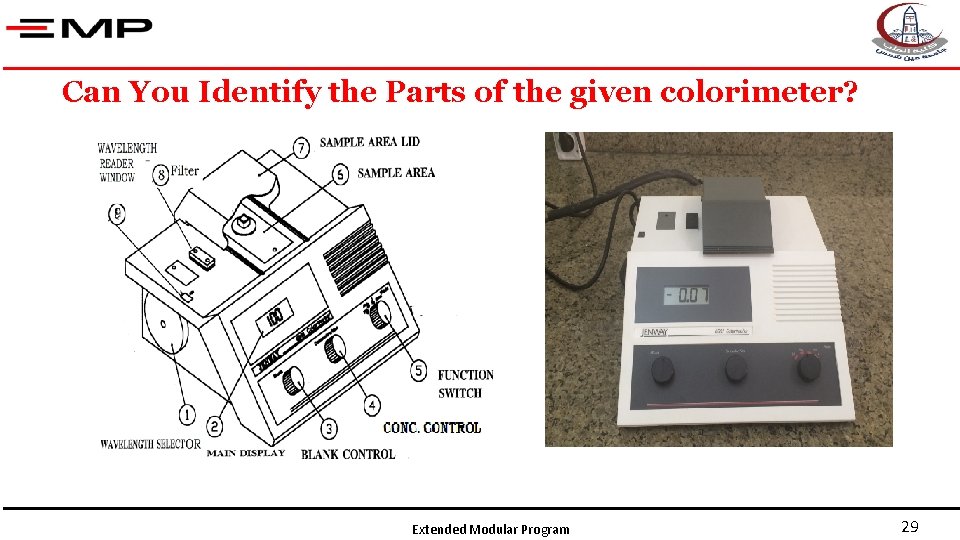
Can You Identify the Parts of the given colorimeter? Extended Modular Program 29
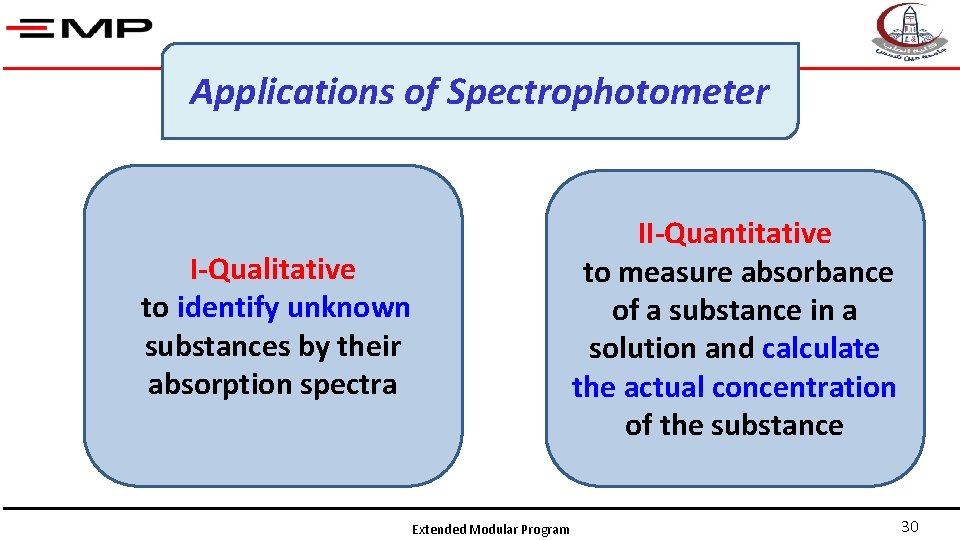
Applications of Spectrophotometer II-Quantitative to measure absorbance of a substance in a solution and calculate the actual concentration of the substance I-Qualitative to identify unknown substances by their absorption spectra Extended Modular Program 30
Qualitative Spectrophotometric assays What is meant by absorption Spectra? • An absorption spectrum is done by measuring the absorbance of the pure substance in a solution at different wavelengths. • Each solution absorbs light maximally at a certain wavelength according to its chemical nature. Extended Modular Program 31
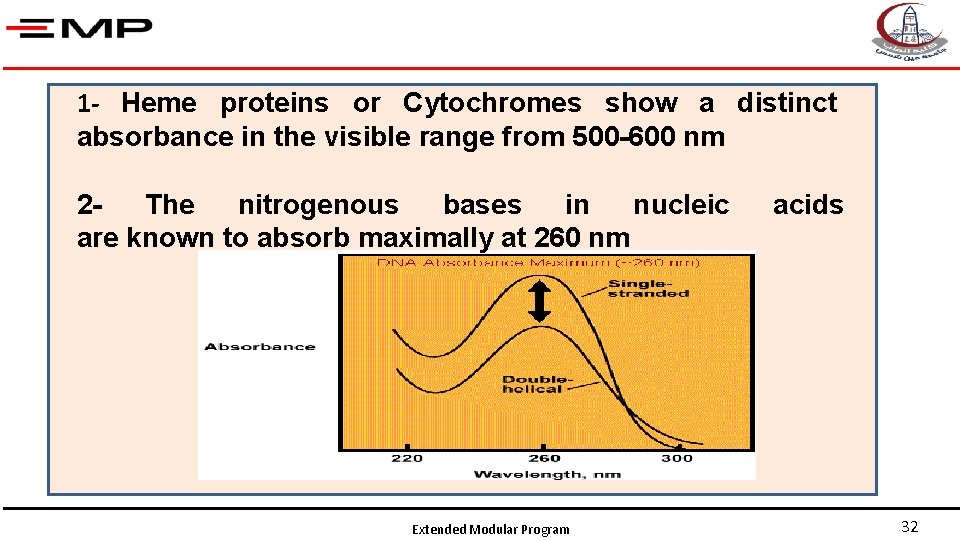
1 - Heme proteins or Cytochromes show a distinct absorbance in the visible range from 500 -600 nm 2 The nitrogenous bases in nucleic are known to absorb maximally at 260 nm Extended Modular Program acids 32
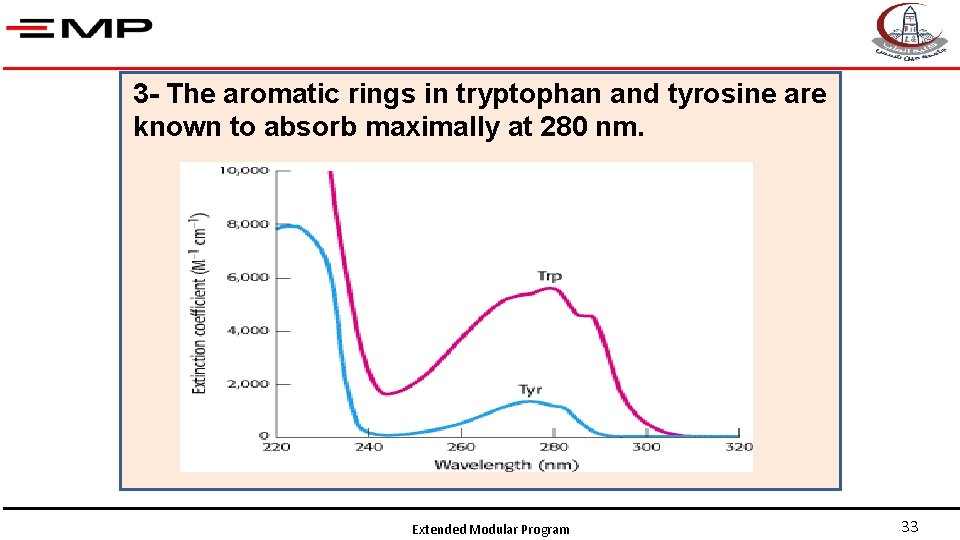
3 - The aromatic rings in tryptophan and tyrosine are known to absorb maximally at 280 nm. Extended Modular Program 33
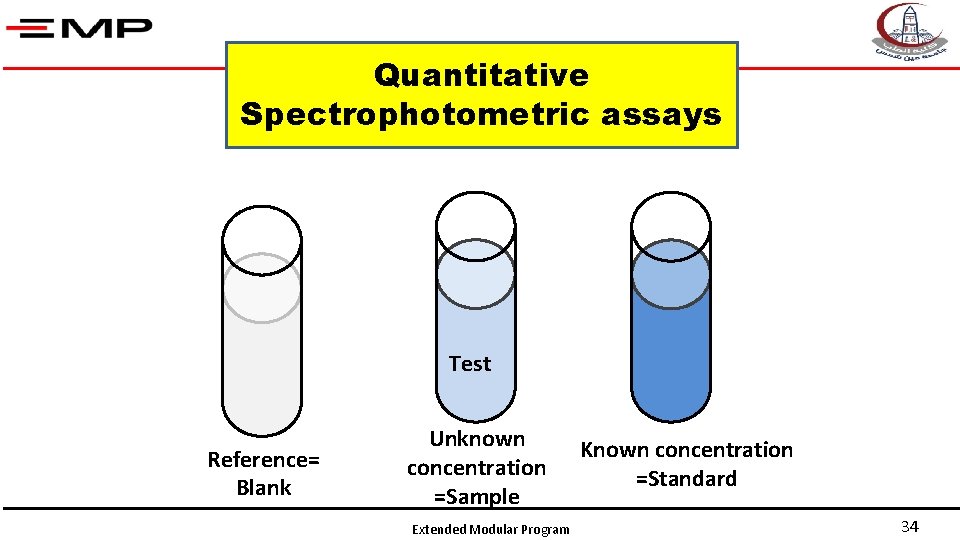
Quantitative Spectrophotometric assays Test Reference= Blank Unknown concentration =Sample Extended Modular Program Known concentration =Standard 34
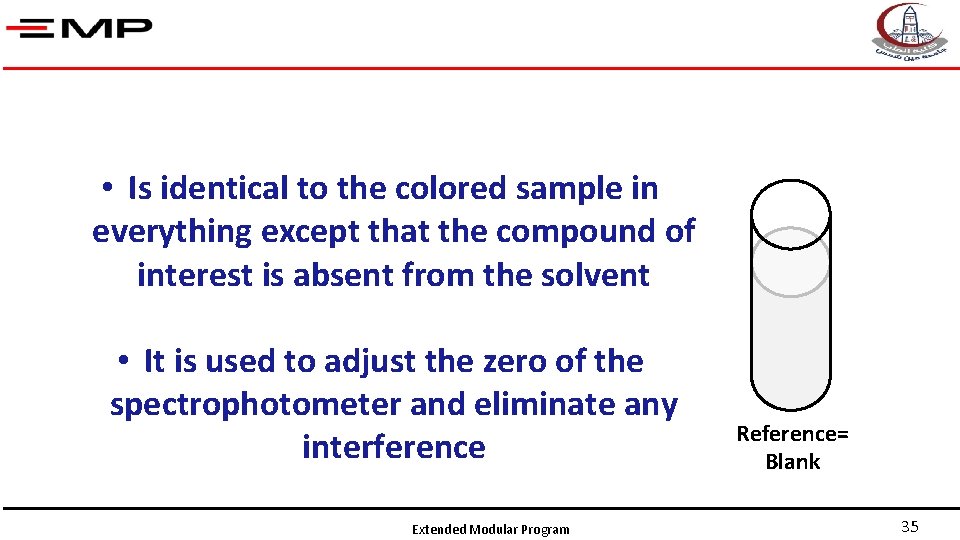
• Is identical to the colored sample in everything except that the compound of interest is absent from the solvent • It is used to adjust the zero of the spectrophotometer and eliminate any interference Extended Modular Program Reference= Blank 35
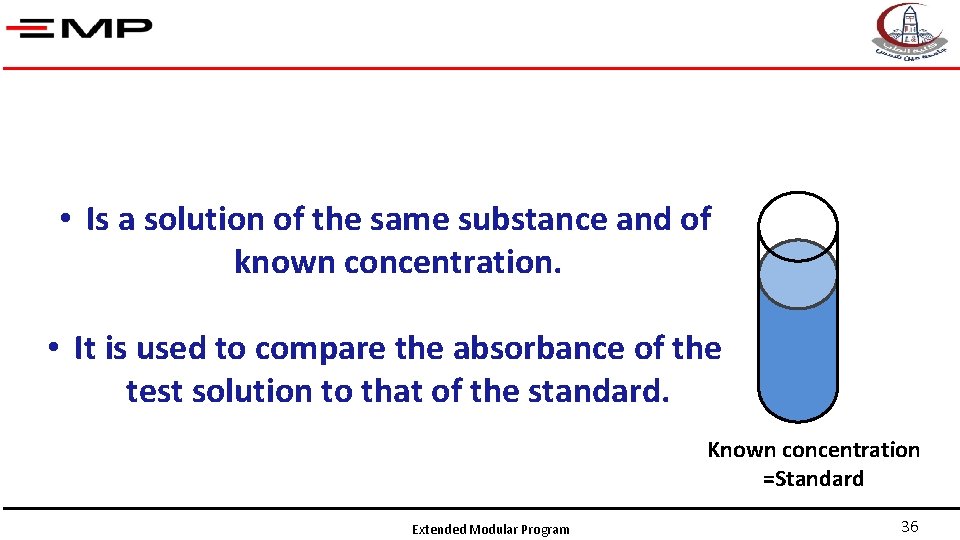
• Is a solution of the same substance and of known concentration. • It is used to compare the absorbance of the test solution to that of the standard. Known concentration =Standard Extended Modular Program 36
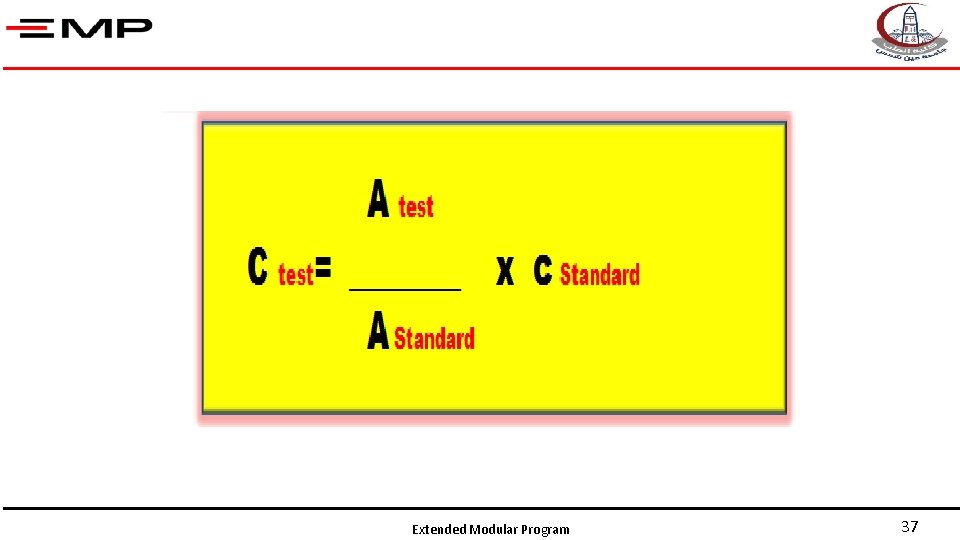
Extended Modular Program 37

STEPS OF USING THE SPECTROPHOTOMETER Extended Modular Program 38

1. Switch On the device by the ON/OFF key at the back. The instrument must be warmed for 15 min. Extended Modular Program 39

2. Set the desired wavelength and check the wavelength window from the top. Extended Modular Program 40

3 - Get a set of clean cuvettes –ready to fill with blank and samples Extended Modular Program 41

4 - Raise the sample area Lid, to be ready for the cuvette insertion. The arrow points to the light path direction. N. B. DO NOT Worry this reading is without a cuvette inserted! Extended Modular Program 42

5 -Ready to insert the BLANK cuvette. N. B. you may wipe the cuvette if needed. 6 - Place the cuvette into the sample holder, then close the cover Extended Modular Program 43

7 - Set the blank by using blank button: • For (A) indicator adjust at Zero absorbance Extended Modular Program 44

8 - Remove blank tube, wipe off the cuvette of sample and insert it, cover the sample area and read the absorbance. Extended Modular Program 45

9 - Record the absorbance then calculate the concentration of the sample, e. g. , protein. Extended Modular Program 46
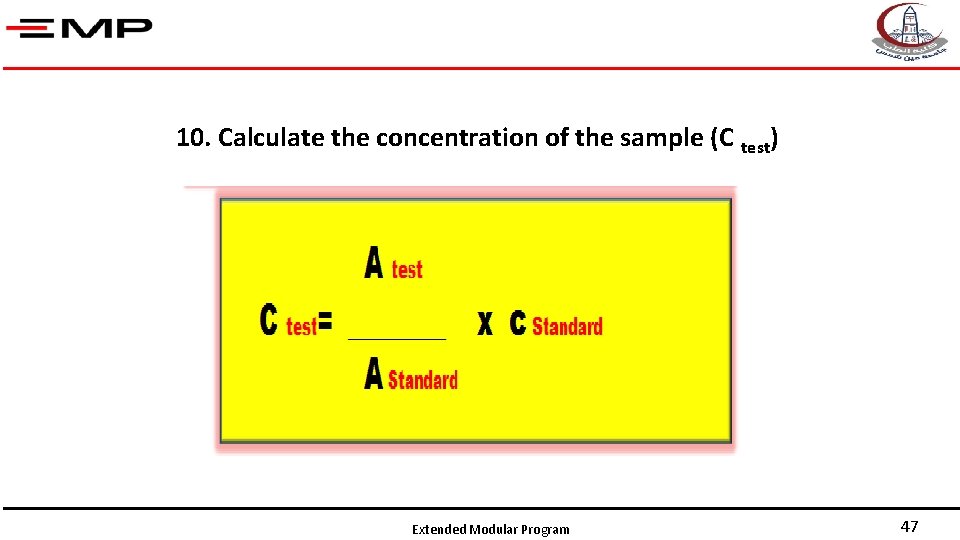
10. Calculate the concentration of the sample (C test) Extended Modular Program 47
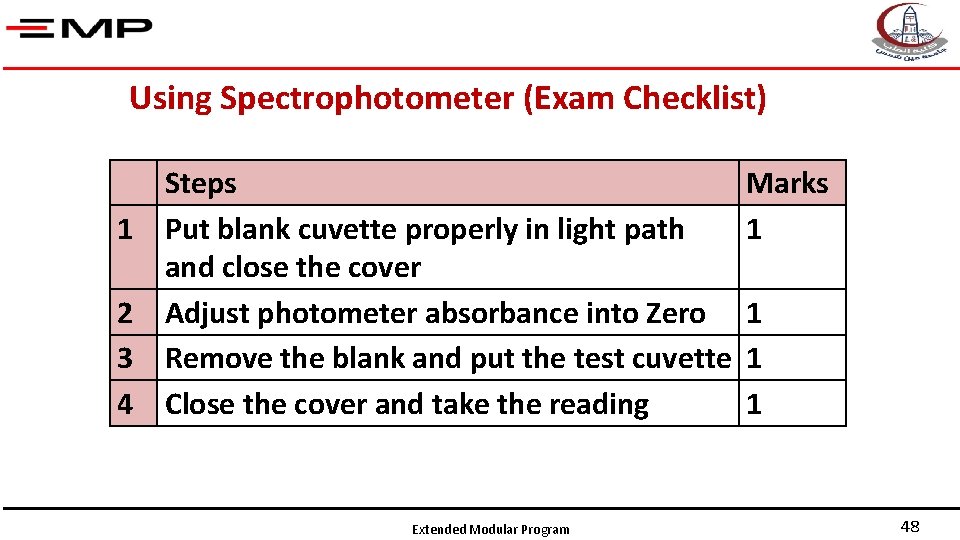
Using Spectrophotometer (Exam Checklist) 1 2 3 4 Steps Put blank cuvette properly in light path and close the cover Adjust photometer absorbance into Zero Remove the blank and put the test cuvette Close the cover and take the reading Extended Modular Program Marks 1 1 48
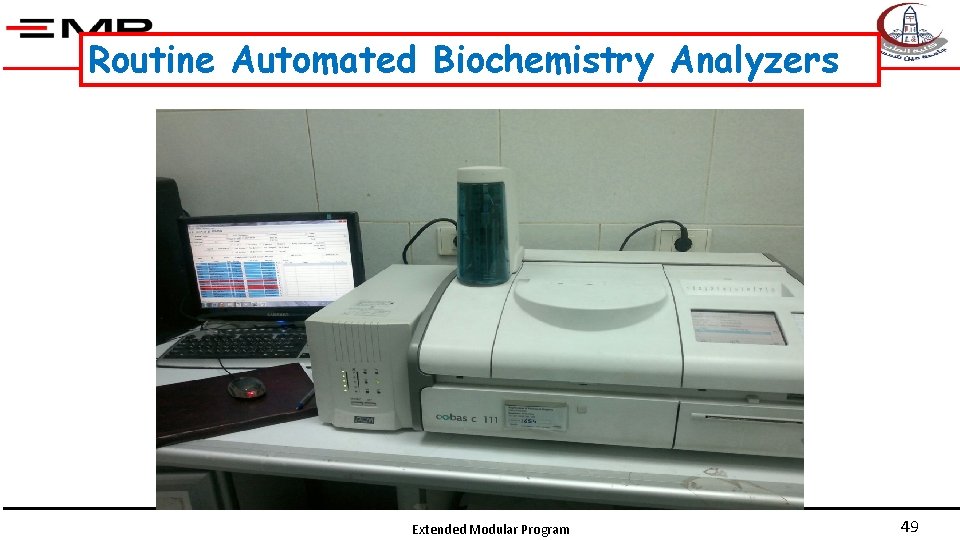
Routine Automated Biochemistry Analyzers Extended Modular Program 49
Routine Automated Biochemistry Analyzers § These are machines that process a large portion of the samples going into a hospital or private medical laboratory. § Automation of the testing process has reduced analytical errors and testing time for many analytes from days to minutes. Extended Modular Program 50
Lab activities Identify photometer with its main parts and uses Recognize the principles of photometry and the related laws (Beer-Lambert’s laws) Demonstrate and practice the steps of using the spectrophotometer to measure analytes

Thank You Extended Modular Program
- Slides: 52