Principles of Pharmacovigilance system introduced by a manufacturer

Principles of Pharmacovigilance system introduced by a manufacturer of medicinal products. A summary of two-year performance of pharmaceutical GVP system

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75

Not simple way to GVP Presence of regulatory act. . . Speaker: Romanov Boris Konstantinovich, M. D. , Deputy Director for Science of FSBI «Scientific Centre for Expert Evaluation of Medicinal Products» of the Ministry of Health of the Russian Federation

Problems of GVP introduction Critical processes: - Pharmacovigilance system master file - Inspections Speaker: Romanov Boris Konstantinovich, M. D. , Deputy Director for Science of FSBI «Scientific Centre for Expert Evaluation of Medicinal Products» of the Ministry of Health of the Russian Federation

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75

EAEU documents including pharmacovigilance issues Union Document Details 1. Guideline on good pharmacovigilance practice in Eurasian Economic Union Council’s decision EEC dated 3. 11. 16 No. 87 2. Requirements for instructions for medical use of drugs and for the general characteristics of drugs for medical use. Annex 1: actions to minimize the risks of excipients Annex 5: actions to minimize the risks during pregnancy and lactation Council’s decision EEC dated 3. 11. 16 No. 88 3. The rules for research of biological drugs in Eurasian Economic Union Paragraph 7 «Pharmacovigilance» Chapters 15. 2 -15. 11: RMPs for biosimilars Council’s decision EEC dated 3. 11. 16 No. 89 Speaker: Rozhdestvensky Dmitry Anatolyevich, Ph. D (Medicine), Technical Regulation and Accreditation Department Eurasian Economic Commission, e-mail: rozhdestvenskiy@eecommission. org, p/n: +7 (495) 669 -2400 (add. 3141)
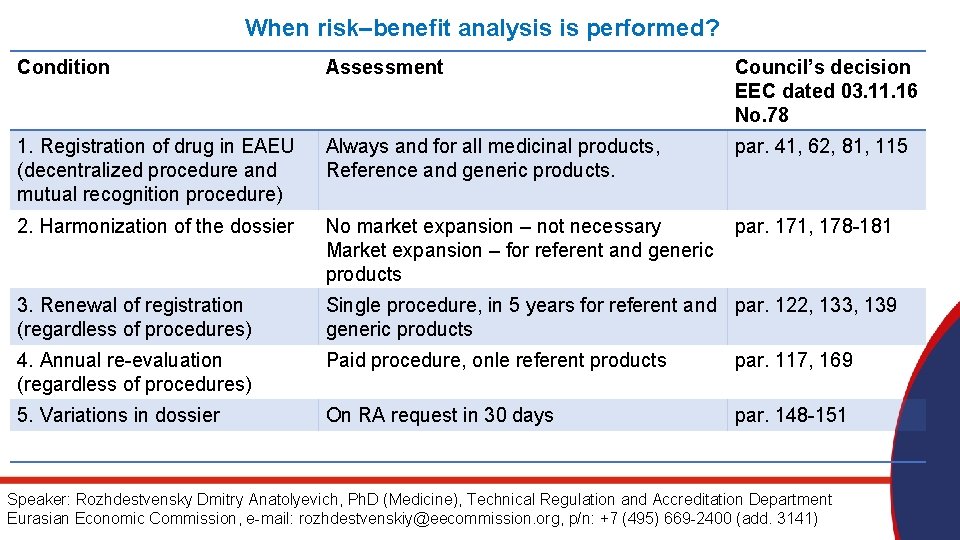
When risk–benefit analysis is performed? Condition Assessment Council’s decision EEC dated 03. 11. 16 No. 78 1. Registration of drug in EAEU (decentralized procedure and mutual recognition procedure) Always and for all medicinal products, Reference and generic products. par. 41, 62, 81, 115 2. Harmonization of the dossier No market expansion – not necessary Market expansion – for referent and generic products par. 171, 178 -181 3. Renewal of registration (regardless of procedures) Single procedure, in 5 years for referent and par. 122, 133, 139 generic products 4. Annual re-evaluation (regardless of procedures) Paid procedure, onle referent products par. 117, 169 5. Variations in dossier On RA request in 30 days par. 148 -151 Speaker: Rozhdestvensky Dmitry Anatolyevich, Ph. D (Medicine), Technical Regulation and Accreditation Department Eurasian Economic Commission, e-mail: rozhdestvenskiy@eecommission. org, p/n: +7 (495) 669 -2400 (add. 3141)
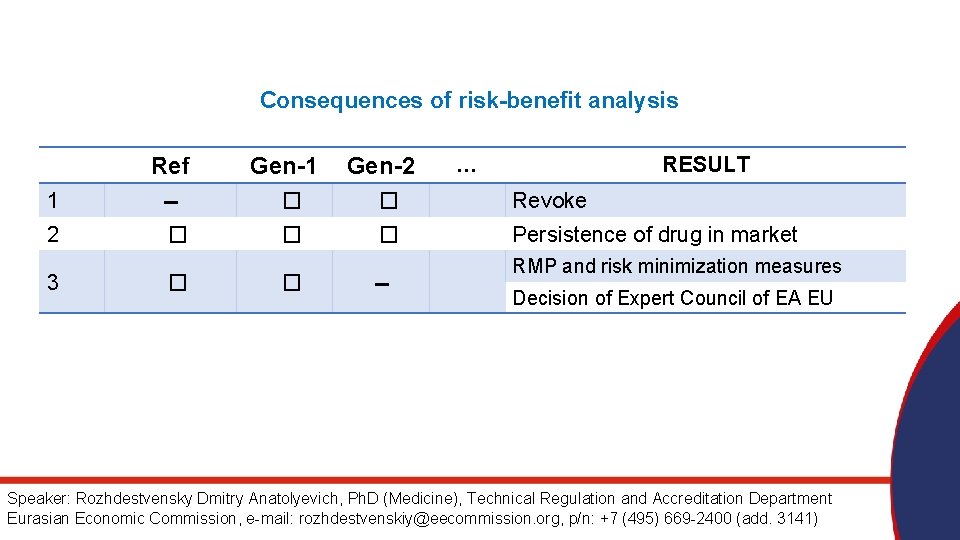
Consequences of risk-benefit analysis Ref Gen-1 Gen-2 … RESULT 1 -- � � Revoke 2 � � � Persistence of drug in market 3 � � -- RMP and risk minimization measures Decision of Expert Council of EA EU Speaker: Rozhdestvensky Dmitry Anatolyevich, Ph. D (Medicine), Technical Regulation and Accreditation Department Eurasian Economic Commission, e-mail: rozhdestvenskiy@eecommission. org, p/n: +7 (495) 669 -2400 (add. 3141)
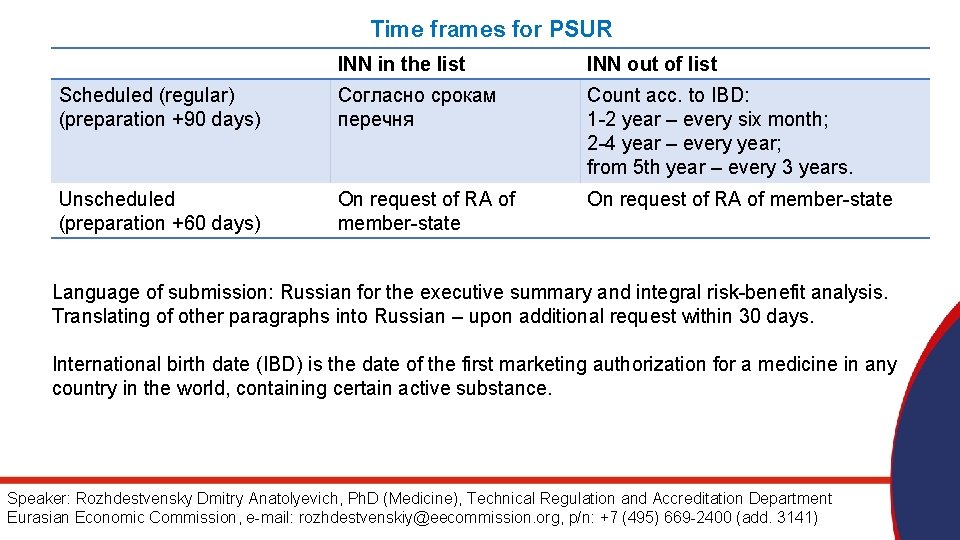
Time frames for PSUR INN in the list INN out of list Scheduled (regular) (preparation +90 days) Согласно срокам перечня Count acc. to IBD: 1 -2 year – every six month; 2 -4 year – every year; from 5 th year – every 3 years. Unscheduled (preparation +60 days) On request of RA of member-state Language of submission: Russian for the executive summary and integral risk-benefit analysis. Translating of other paragraphs into Russian – upon additional request within 30 days. International birth date (IBD) is the date of the first marketing authorization for a medicine in any country in the world, containing certain active substance. Speaker: Rozhdestvensky Dmitry Anatolyevich, Ph. D (Medicine), Technical Regulation and Accreditation Department Eurasian Economic Commission, e-mail: rozhdestvenskiy@eecommission. org, p/n: +7 (495) 669 -2400 (add. 3141)

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75
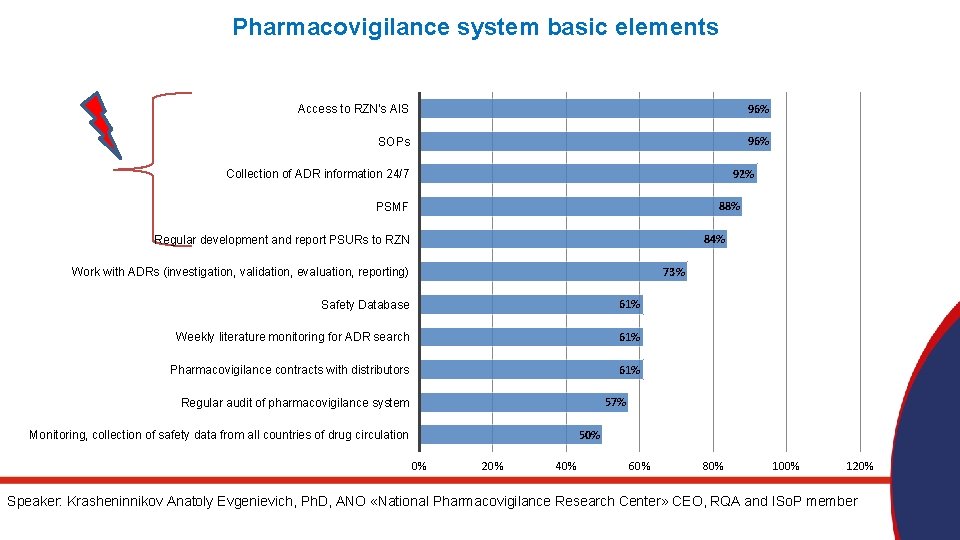
Pharmacovigilance system basic elements Access to RZN's AIS 96% SOPs 96% 92% Collection of ADR information 24/7 88% PSMF 84% Regular development and report PSURs to RZN 73% Work with ADRs (investigation, validation, evaluation, reporting) Safety Database 61% Weekly literature monitoring for ADR search 61% Pharmacovigilance contracts with distributors 61% 57% Regular audit of pharmacovigilance system 50% Monitoring, collection of safety data from all countries of drug circulation 0% 20% 40% 60% 80% 100% 120% Speaker: Krasheninnikov Anatoly Evgenievich, Ph. D, ANO «National Pharmacovigilance Research Center» CEO, RQA and ISo. P member

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75
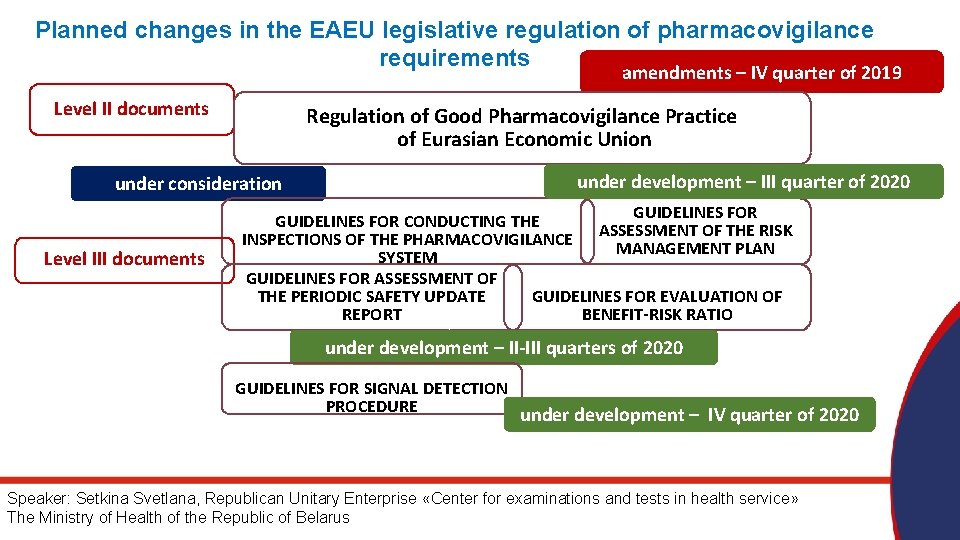
Planned changes in the EAEU legislative regulation of pharmacovigilance requirements amendments – IV quarter of 2019 Level II documents Regulation of Good Pharmacovigilance Practice of Eurasian Economic Union under development – III quarter of 2020 under consideration Level III documents GUIDELINES FOR CONDUCTING THE OF THE RISK INSPECTIONS OF THE PHARMACOVIGILANCE ASSESSMENT MANAGEMENT PLAN SYSTEM GUIDELINES FOR ASSESSMENT OF THE PERIODIC SAFETY UPDATE GUIDELINES FOR EVALUATION OF REPORT BENEFIT-RISK RATIO under development – II-III quarters of 2020 GUIDELINES FOR SIGNAL DETECTION PROCEDURE under development – IV quarter of 2020 Speaker: Setkina Svetlana, Republican Unitary Enterprise «Center for examinations and tests in health service» The Ministry of Health of the Republic of Belarus
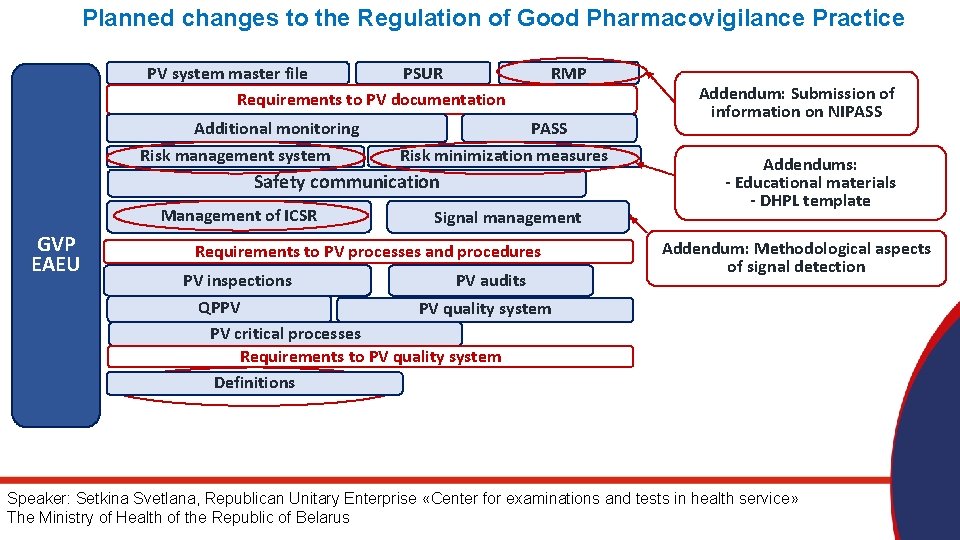
Planned changes to the Regulation of Good Pharmacovigilance Practice PSUR PV system master file Requirements to PV documentation Additional monitoring Risk management system RMP PASS Risk minimization measures Safety communication Management of ICSR GVP EAEU Signal management Requirements to PV processes and procedures PV inspections PV audits QPPV PV quality system PV critical processes Requirements to PV quality system Definitions Addendum: Submission of information on NIPASS Addendums: - Educational materials - DHPL template Addendum: Methodological aspects of signal detection Speaker: Setkina Svetlana, Republican Unitary Enterprise «Center for examinations and tests in health service» The Ministry of Health of the Republic of Belarus

GVP inspection throughout the life cycle of medicinal products MAH has not previously operated a pharmacovigilance within the EAEU / in the process of establishing a new pharmacovigilance system Available information (from RA) indicates that the MAH has a poor history of compliance PV system requirements GVP Product-specific safety concerns (e. g. , complication with inspection possibility to implement RMP, product-specific risk minimisation additional and routine activity) Development Authorisation Pre-authorization period Not mandatory, BUT RA can conduct PV inspection before marketing authorisation is granted for examining of compliance of MAH PV system (existed or planned) to legislation and GVP requirements Risk-based approach Routine inspections For-cause inspection (PV inspection triggers) GVP inspection Post-authorisation use Post-authorization period RA can conduct PV inspection in any time of life cycle of medicinal product RA: - publishes annual PV inspection plan - Initiates “for-cause” PV inspection procedure when triggers are identified Speaker: Setkina Svetlana, Republican Unitary Enterprise «Center for examinations and tests in health service» The Ministry of Health of the Republic of Belarus
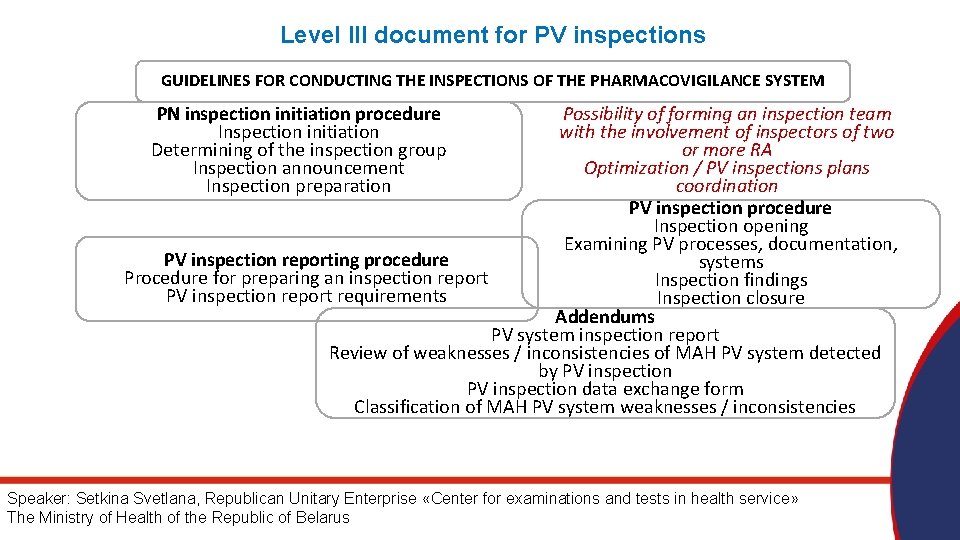
Level III document for PV inspections GUIDELINES FOR CONDUCTING THE INSPECTIONS OF THE PHARMACOVIGILANCE SYSTEM PN inspection initiation procedure Inspection initiation Determining of the inspection group Inspection announcement Inspection preparation Possibility of forming an inspection team with the involvement of inspectors of two or more RA Optimization / PV inspections plans coordination PV inspection procedure Inspection opening Examining PV processes, documentation, PV inspection reporting procedure systems Procedure for preparing an inspection report Inspection findings PV inspection report requirements Inspection closure Addendums PV system inspection report Review of weaknesses / inconsistencies of MAH PV system detected by PV inspection data exchange form Classification of MAH PV system weaknesses / inconsistencies Speaker: Setkina Svetlana, Republican Unitary Enterprise «Center for examinations and tests in health service» The Ministry of Health of the Republic of Belarus

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75

Regulatory documents • Code of the Republic of Kazakhstan dated 18 September 2009 «On People's Health and Healthcare System» - Article 85 «Pharmacovigilance and monitoring of the safety, quality and effectiveness of medical devices» • Order of the Ministry of Healthcare and Social Development of the Republic of Kazakhstan No. 392 dated 27 May 2015 «On approval Gx. P» Annex 6 (Good Pharmacovigilance Practice - GVP) • Order of the Ministry of Healthcare and Social Development of the Republic of Kazakhstan No. 421 dated 29 May 2015 «Rules for conducting pharmacovigilance of medicines and monitoring side effects of medicines, medical devices and medical equipment» • Order of Ministry of Healthcare and Social Development of the Republic of Kazakhstan No. 106 dated 27 February 2015 «On approval of the Rules of the prohibition, suspension or withdrawal from circulation of medicines, medical devices and medical equipment» • Order of the Health Minister of the Republic of Kazakhstan No. 701 dated 12 November 2009 «On approval of the Rules for the withdrawal of samples of medicines, medical devices and medical equipment for expertise» • Guidelines on good pharmacovigilance practices of Eurasian Economic Union approved by Council’s decision of the Eurasian Economic Commission No. 87 dated 03 November 2016; Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28
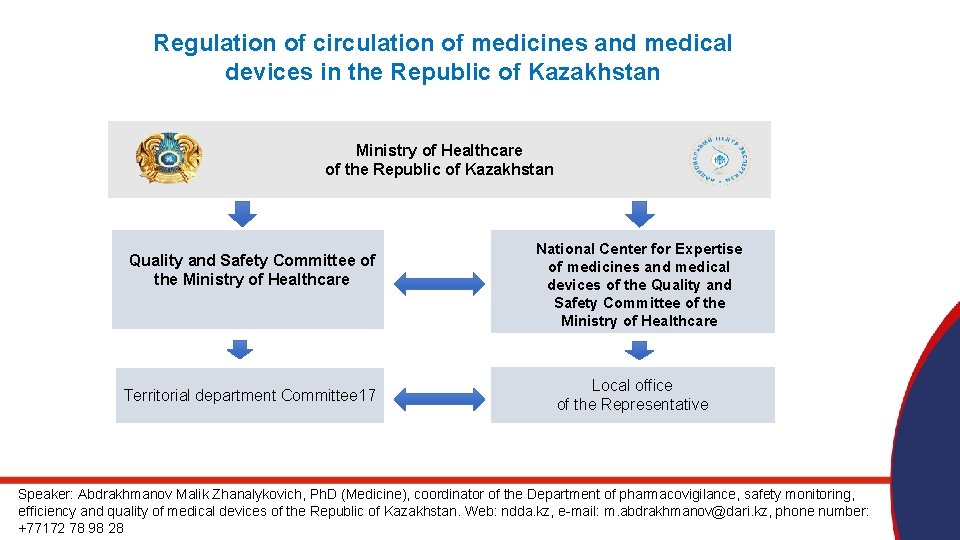
Regulation of circulation of medicines and medical devices in the Republic of Kazakhstan Ministry of Healthcare of the Republic of Kazakhstan Quality and Safety Committee of the Ministry of Healthcare Territorial department Committee 17 National Center for Expertise of medicines and medical devices of the Quality and Safety Committee of the Ministry of Healthcare Local office of the Representative Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28

Interaction system Quality and Safety Committee of the Ministry of Healthcare Information on side effects of drugs Regulatory actions • Report serious adverse reactions (death, life threatening, lack of effectiveness, abuse, offlabel use, unregistered drugs) within 48 hours. • Conclusions about casuality and benefit-risk profile. • Withdrawal of samples • Prohibition of medical use and withdrawal from circulation or suspension of medical use of lot of drugs Amending the instructions for medical use • Appointment of a MAH inspection • Suspension of action/Recall of the marketing authorisation Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28

Pharmacovigilance system: 1 2 Collection and Analysis, verification of assessment and expertise of reports of drug safety data ADRs from MAH 3 4 5 6 Monitoring drug safety data from other sources Signal evaluation based on a database for monitoring side effects of drugs Annual assessment of benefit-risk profile of drugs Regulatory actions Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28
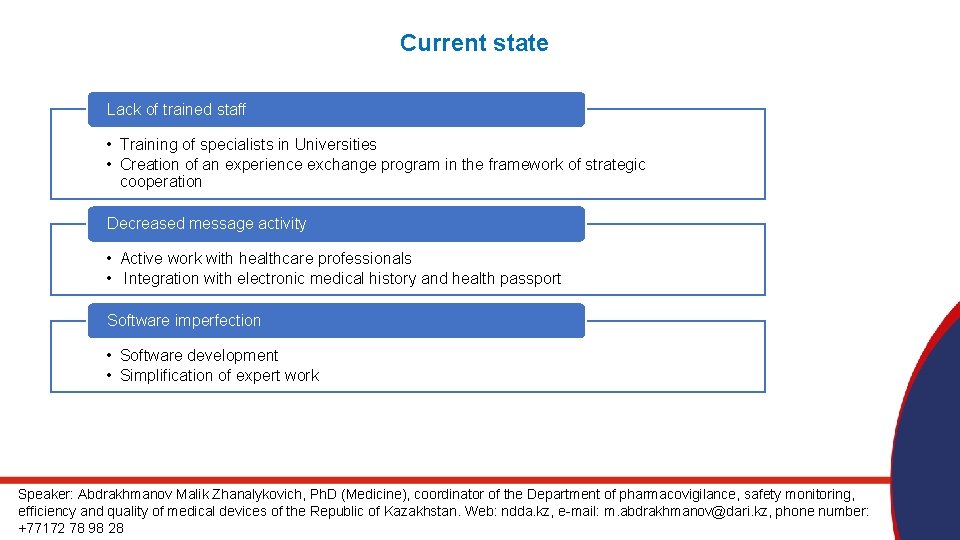
Current state Lack of trained staff • Training of specialists in Universities • Creation of an experience exchange program in the framework of strategic cooperation Decreased message activity • Active work with healthcare professionals • Integration with electronic medical history and health passport Software imperfection • Software development • Simplification of expert work Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28

Prospects Re-organization • Creation of new units • Involvement of outside specialists, industry experts Introduction • New technological solutions • Pharmacoepidemiology Development • risk-oriented approach • Integration in EAEU Pharmacovigilance system • Preparation of the Bachelor Degree Program, Ph. D (Pharmacovigilance) Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28
Speaker: Abdrakhmanov Malik Zhanalykovich, Ph. D (Medicine), coordinator of the Department of pharmacovigilance, safety monitoring, efficiency and quality of medical devices of the Republic of Kazakhstan. Web: ndda. kz, e-mail: m. abdrakhmanov@dari. kz, phone number: +77172 78 98 28

You can address your questions to speakers via Whats. App +7 (967)-374 -71 -75

How prepared are Kazakhstan drug companies for inspections? The results of a small anonymous survey of Kazakhstan pharmaceutical manufacturers* showed that: • All manufacturing companies carefully follow the legislation regarding the organization and conduct of pharmacovigilance. • All surveyed companies conducted an internal audit of the pharmacovigilance system. • Only a few have passed inspection by the regulator (successful). *was surveyed: • large companies with a huge assortment portfolio (3); • medium companies with a small portfolio of widely used products(4); • small companies with a limited range of medicines(3). Speaker: Durmanova Marina, president of Pharmaceutical Support and Development Association of Republic of Kazakhstan

The main difficulties in organizing the pharmacovigilance system currently experienced by pharmaceutical manufacturers in Republic of Kazakhstan Among the difficulties local manufacturers called: • The shortage of personnel who have experience in the field of pharmacovigilance; • Combining the QPPV position with other positions (typical for small companies, large and medium-sized enterprises have entire departments); • Low involvement of medical workers and pharmacists; • Low patient awareness of the need to report side effects of drugs. Misunderstanding of the importance of this work; • Lack of loyalty of heads of healthcare organizations, if necessary, to respond to company inquiries when investigating cases of side effects of drugs; • The presence of fears among company leaders that the identification of cases of side effects of drugs may adversely affect the image of the enterprise. Speaker: Durmanova Marina, president of Pharmaceutical Support and Development Association of Republic of Kazakhstan

Among the positive factors QPPVs noted: • Professionality and involvement of representatives of the expert body; • Conducting pharmacovigilance training cycles as part of pre- and postgraduate training programs involving QPPV from pharmaceutical companies; • Enhanced ability to submit reports through the online resource of an expert organization online, by fax, email or on paper. QPPVs called necessary: • Increasing Primary Health Care's involvement in monitoring drug side effects; • Conducting more extensive training programs for medical and pharmaceutical workers (the latter have been recently involved in the pharmacovigilance system, not all pharmacies have trained personnel); • The need for more active educational work among the population. Speaker: Durmanova Marina, president of Pharmaceutical Support and Development Association of Republic of Kazakhstan

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75
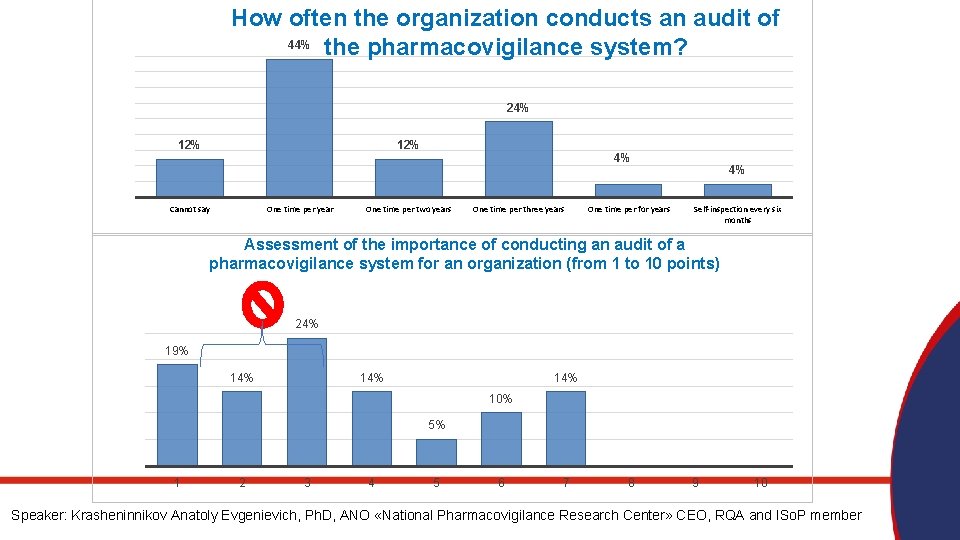
How often the organization conducts an audit of 44% the pharmacovigilance system? 24% 12% Cannot say One time per year 4% One time per two years One time per three years One time per for years 4% Self-inspection every six months Assessment of the importance of conducting an audit of a pharmacovigilance system for an organization (from 1 to 10 points) 24% 19% 14% 14% 10% 5% 1 2 3 4 5 6 7 8 9 10 Speaker: Krasheninnikov Anatoly Evgenievich, Ph. D, ANO «National Pharmacovigilance Research Center» CEO, RQA and ISo. P member
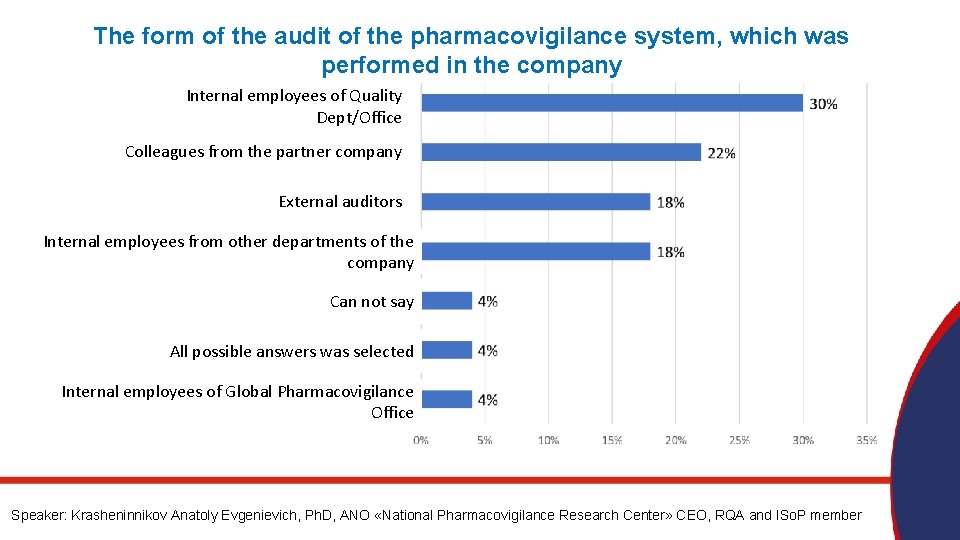
The form of the audit of the pharmacovigilance system, which was performed in the company Internal employees of Quality Dept/Office Colleagues from the partner company External auditors Internal employees from other departments of the company Can not say All possible answers was selected Internal employees of Global Pharmacovigilance Office Speaker: Krasheninnikov Anatoly Evgenievich, Ph. D, ANO «National Pharmacovigilance Research Center» CEO, RQA and ISo. P member

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75
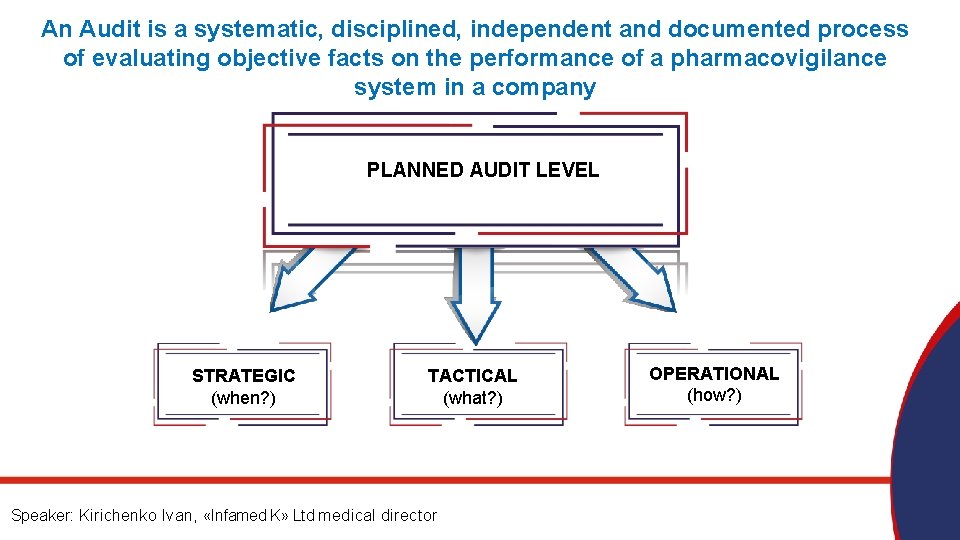
An Audit is a systematic, disciplined, independent and documented process of evaluating objective facts on the performance of a pharmacovigilance system in a company PLANNED AUDIT LEVEL STRATEGIC (when? ) TACTICAL (what? ) Speaker: Kirichenko Ivan, «Infamed K» Ltd medical director OPERATIONAL (how? )

PHARMACOVIGILANCE COVERS ALL THE ELEMENTS IN A COMPANY’S ORGANIZATIONAL STRUCTURE Medical Pharmacovigilance PHARMACOVIGILANCE Registration R&D Pharmaceuticals Sales Marketing IT HR Distributors Speaker: Kirichenko Ivan, «Infamed K» Ltd medical director

ROLE OF DISTRIBUTORS: source of information about drugs efficiency, safety and quality MAH Pharmacovigilance Distributor Дистрибьютор Speaker: Kirichenko Ivan, «Infamed K» Ltd medical director Pharmacies Аптеки Конечный End consumer потребитель

You can address your questions/comments to speakers via Whats. App +7 (967)-374 -71 -75
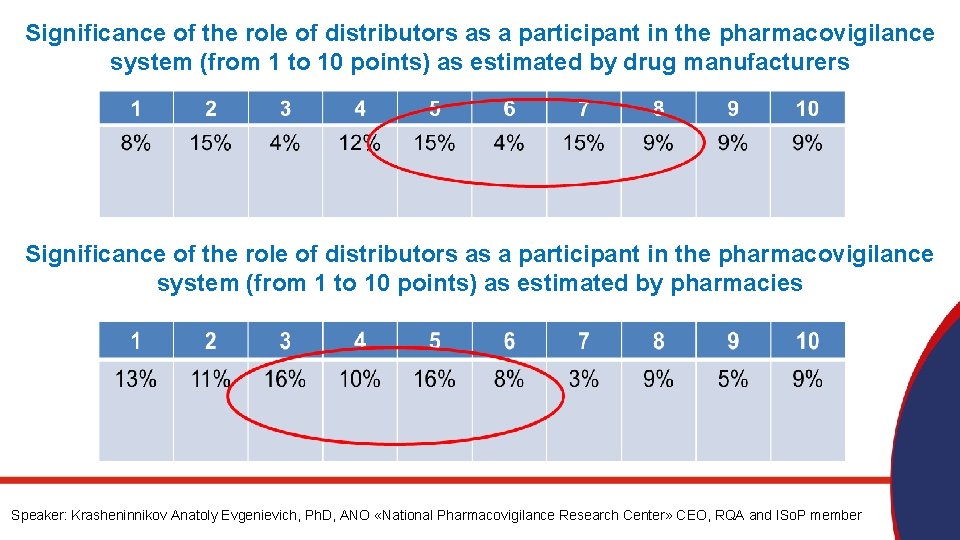
Significance of the role of distributors as a participant in the pharmacovigilance system (from 1 to 10 points) as estimated by drug manufacturers Significance of the role of distributors as a participant in the pharmacovigilance system (from 1 to 10 points) as estimated by pharmacies Speaker: Krasheninnikov Anatoly Evgenievich, Ph. D, ANO «National Pharmacovigilance Research Center» CEO, RQA and ISo. P member

ROLE OF QPPV IN COMPANY STRUCTURE Chief Executive Officer Director-at-large Medical Director Commercial director QPPV LQPPV Speaker: Krasheninnikov Anatoly Evgenievich, Ph. D, ANO «National Pharmacovigilance Research Center» CEO, RQA and ISo. P member

You can address your questions to speakers via Whats. App +7 (967)-374 -71 -75
- Slides: 39