Principles of gas detection Catalytic Electrochemical cell Infrared


- Slides: 32

Principles of gas detection Catalytic, Electrochemical cell, Infrared You know you’re in the Zone™

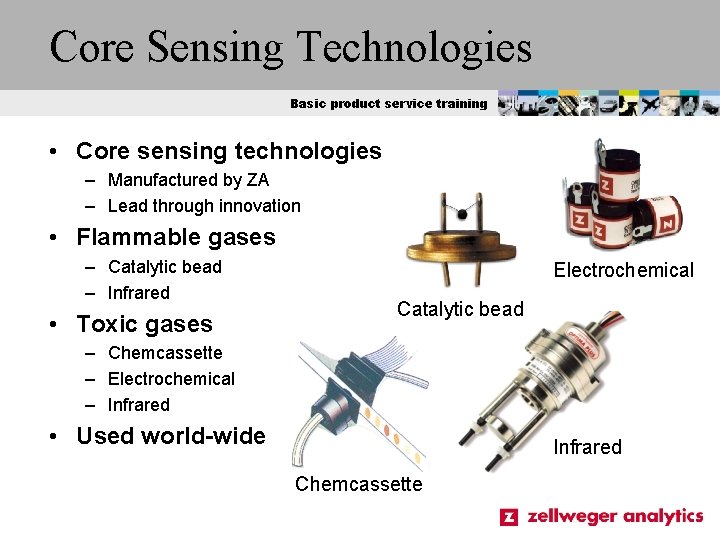
Core Sensing Technologies Basic product service training • Core sensing technologies – Manufactured by ZA – Lead through innovation • Flammable gases – Catalytic bead – Infrared • Toxic gases Electrochemical Catalytic bead – Chemcassette – Electrochemical – Infrared • Used world-wide Infrared Chemcassette

Gas Hazards Basic product service training There are three main types of gas hazard 1. Flammable – Risk of fire and or explosion, e. g. Methane, Butane, Propane 2. Toxic – Risk of poisoning, e. g. Carbon Monoxide, Hydrogen Sulfide, Chlorine 3. Asphyxiant – Risk of suffocation, e. g. Oxygen deficiency, Nitrogen, Carbon Dioxide

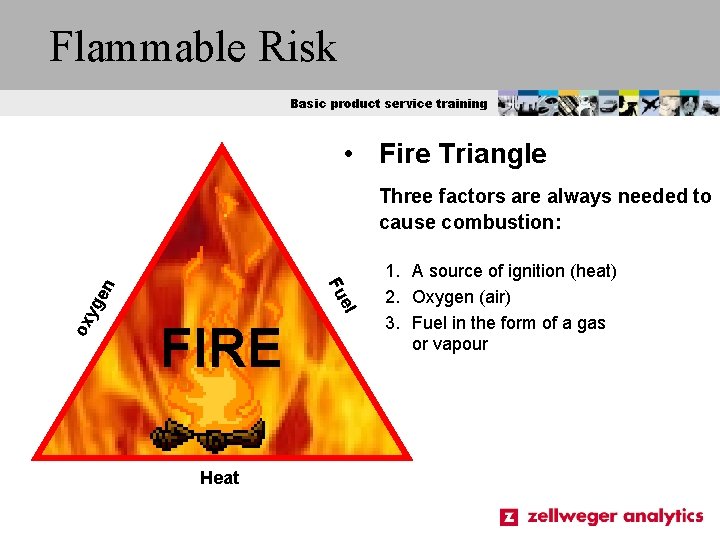
Flammable Risk Basic product service training • Fire Triangle Three factors are always needed to cause combustion: ox l yg en e Fu FIRE Heat 1. A source of ignition (heat) 2. Oxygen (air) 3. Fuel in the form of a gas or vapour

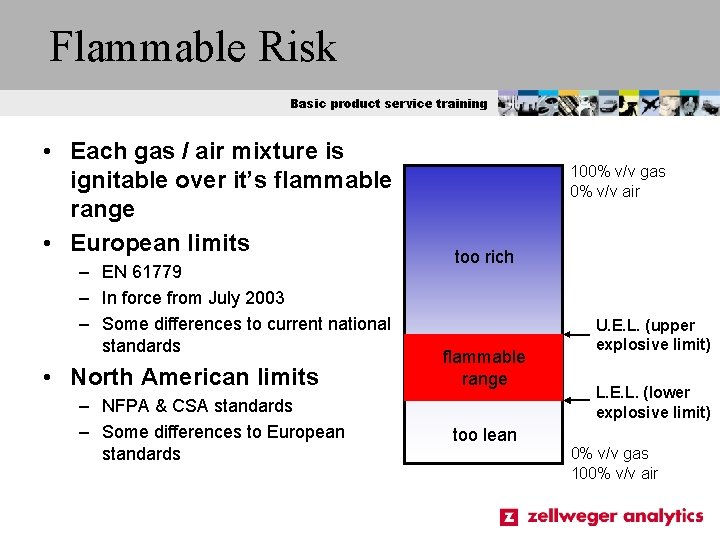
Flammable Risk Basic product service training • Each gas / air mixture is ignitable over it’s flammable range • European limits – EN 61779 – In force from July 2003 – Some differences to current national standards • North American limits – NFPA & CSA standards – Some differences to European standards 100% v/v gas 0% v/v air too rich flammable range U. E. L. (upper explosive limit) L. E. L. (lower explosive limit) too lean 0% v/v gas 100% v/v air

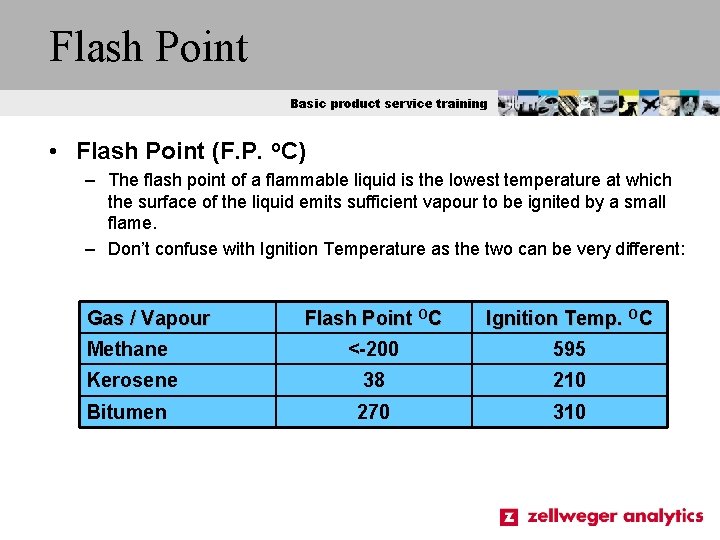
Flash Point Basic product service training • Flash Point (F. P. o. C) – The flash point of a flammable liquid is the lowest temperature at which the surface of the liquid emits sufficient vapour to be ignited by a small flame. – Don’t confuse with Ignition Temperature as the two can be very different: Gas / Vapour Flash Point OC Ignition Temp. OC Methane <-200 595 Kerosene 38 210 Bitumen 270 310

Ignition Temperature Basic product service training • Flammable gases also have a temperature where ignition will take place, even without an external ignition source such as a spark or flame • This temperature is called the Ignition Temperature • Apparatus for use in a hazardous area must does not have a surface temperature that exceeds the ignition temperature • Apparatus is therefore marked with a maximum surface temperature or T rating

Toxic Risk Basic product service training • Some gases are poisonous and can be dangerous to life at very low concentrations. • Some toxic gases have strong smells like the distinctive ‘rotten eggs’ smell of H 2 S • Others are completely odourless like Carbon Monoxide

Toxic gas limits & terminology • Time Weighted Average (TWA) Basic product service training – Toxic gas limits related to concentration & time • Short Term Exposure Limit (STEL) – The maximum allowable concentration over 10 minutes. • Long Term Exposure Limit (LTEL) – The maximum allowable concentration over an 8 hour period. • Units of measure – Parts per million (ppm) – Milligrams per cubic metre (mg/m 3) • Levels – COSHH – OSHA, NIOSH

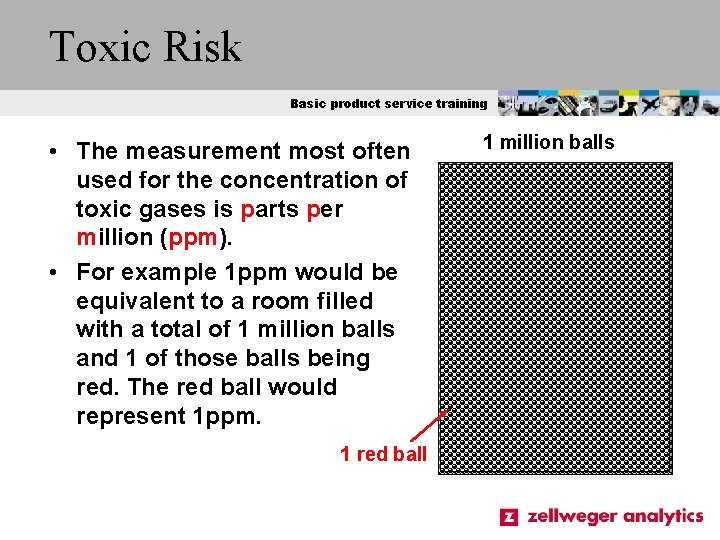
Toxic Risk Basic product service training • The measurement most often used for the concentration of toxic gases is parts per million (ppm). • For example 1 ppm would be equivalent to a room filled with a total of 1 million balls and 1 of those balls being red. The red ball would represent 1 ppm. 1 red ball 1 million balls

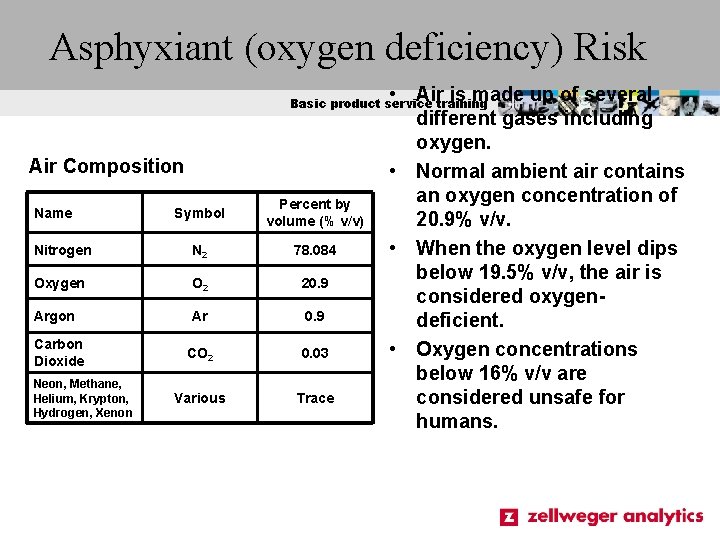
Asphyxiant (oxygen deficiency) Risk • Air is made up of several different gases including oxygen. • Normal ambient air contains an oxygen concentration of 20. 9% v/v. • When the oxygen level dips below 19. 5% v/v, the air is considered oxygendeficient. • Oxygen concentrations below 16% v/v are considered unsafe for humans. Basic product service training Air Composition Symbol Percent by volume (% v/v) Nitrogen N 2 78. 084 Oxygen O 2 20. 9 Argon Ar 0. 9 CO 2 0. 03 Various Trace Name Carbon Dioxide Neon, Methane, Helium, Krypton, Hydrogen, Xenon

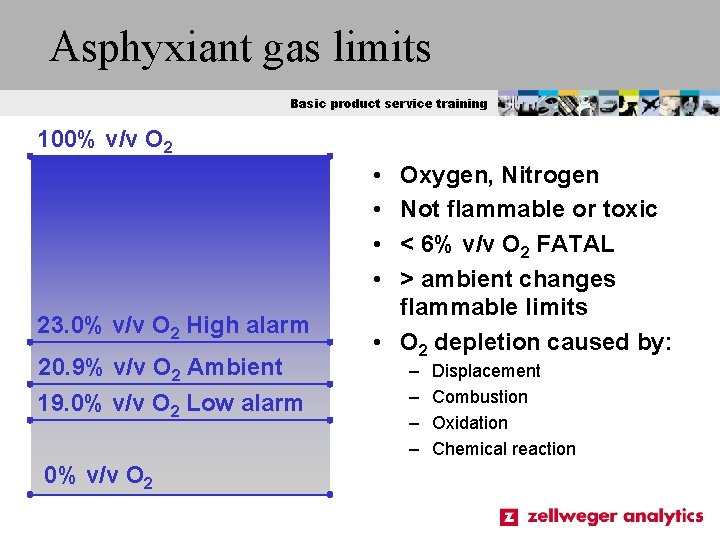
Asphyxiant gas limits Basic product service training 100% v/v O 2 • • 23. 0% v/v O 2 High alarm 20. 9% v/v O 2 Ambient 19. 0% v/v O 2 Low alarm 0% v/v O 2 Oxygen, Nitrogen Not flammable or toxic < 6% v/v O 2 FATAL > ambient changes flammable limits • O 2 depletion caused by: – – Displacement Combustion Oxidation Chemical reaction

Oxygen Enrichment Basic product service training • It is often forgotten that Oxygen enrichment can also cause a risk. • At increased O 2 levels the flammability of materials and gases increases. • At levels of 24% items such as clothing can spontaneously combust. • Oxyacetylene welding equipment combines oxygen and acetylene gas to produce an extremely high temperature. • Leaks from the O 2 cylinders is the main hazard. • Sensors have to be specially certified for use in O 2 enriched atmospheres.

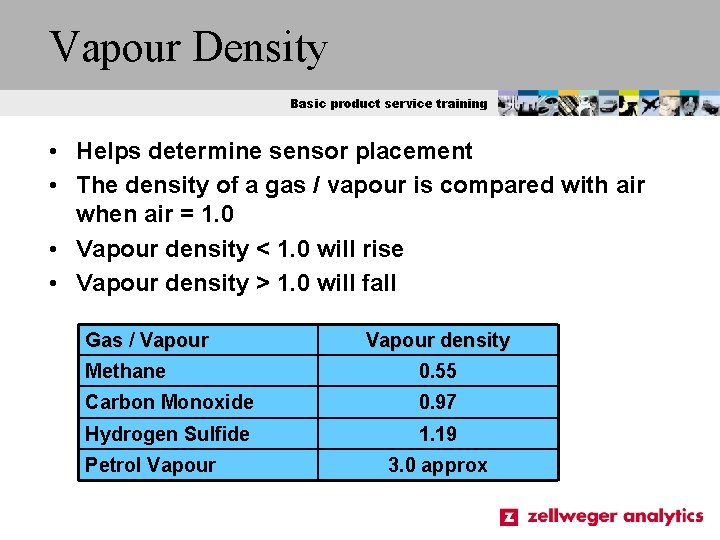
Vapour Density Basic product service training • Helps determine sensor placement • The density of a gas / vapour is compared with air when air = 1. 0 • Vapour density < 1. 0 will rise • Vapour density > 1. 0 will fall Gas / Vapour density Methane 0. 55 Carbon Monoxide 0. 97 Hydrogen Sulfide 1. 19 Petrol Vapour 3. 0 approx

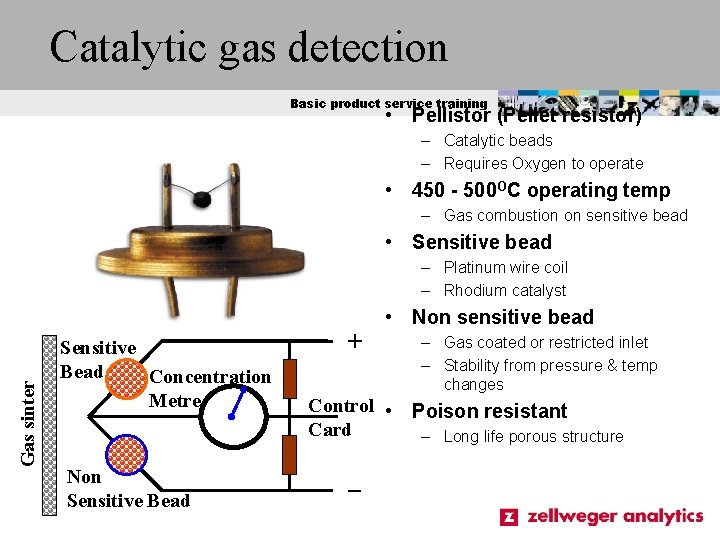
Catalytic gas detection Basic product service training • Pellistor (Pellet resistor) – Catalytic beads – Requires Oxygen to operate • 450 - 500 OC operating temp – Gas combustion on sensitive bead • Sensitive bead – Platinum wire coil – Rhodium catalyst Gas sinter • Non sensitive bead Sensitive Bead Concentration Metre Non Sensitive Bead + – Gas coated or restricted inlet – Stability from pressure & temp changes Control • Poison resistant Card – Long life porous structure _

Catalytic gas detection pros & cons Basic product service training • Speed of response – 10 -20 seconds (T 90) • Sensitivity – 0 -20 & 0 -100% LEL options • Not fail safe – Poisoned by: sulphurs, silicones, phosphors & Leads – Inhibited by: Chlorinated & Fluorinated hydrocarbons • Low powered – Typically 200 m. A allows reduced battery back up • Costs – Low initial cost – High routine maintenance costs

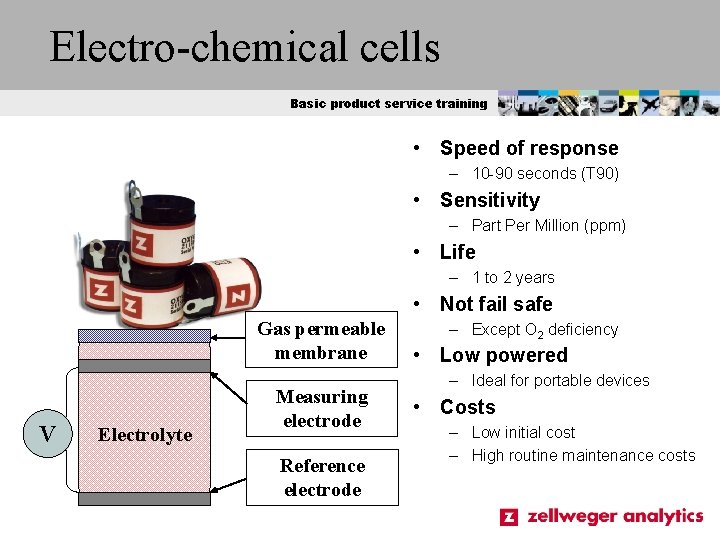
Electro-chemical cells Basic product service training • Speed of response – 10 -90 seconds (T 90) • Sensitivity – Part Per Million (ppm) • Life – 1 to 2 years • Not fail safe Gas permeable membrane V Electrolyte Measuring electrode Reference electrode – Except O 2 deficiency • Low powered – Ideal for portable devices • Costs – Low initial cost – High routine maintenance costs

Electro-chemical cells pros & cons Basic product service training • Speed of response – 10 -90 seconds (T 90) • Sensitivity – Part Per Million (ppm) • Life – 1 to 2 years • Not fail safe – Except O 2 deficiency • Low powered – Ideal for portable devices • Costs – Low initial cost – High routine maintenance costs

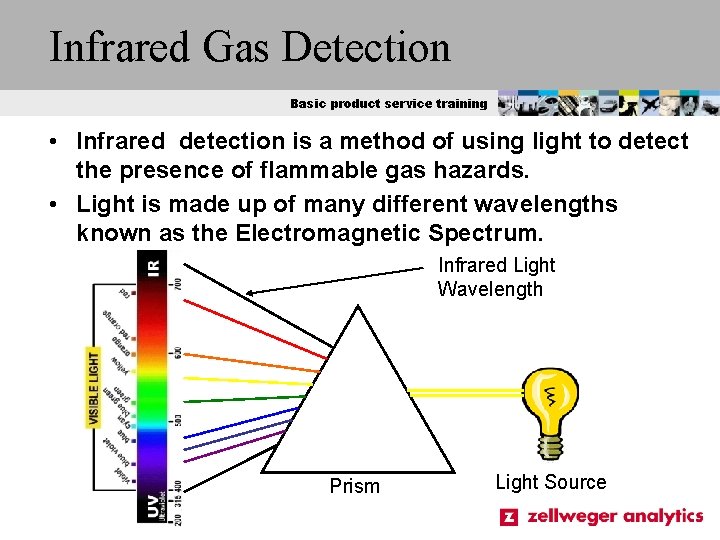
Infrared Gas Detection Basic product service training • Infrared detection is a method of using light to detect the presence of flammable gas hazards. • Light is made up of many different wavelengths known as the Electromagnetic Spectrum. Infrared Light Wavelength Prism Light Source

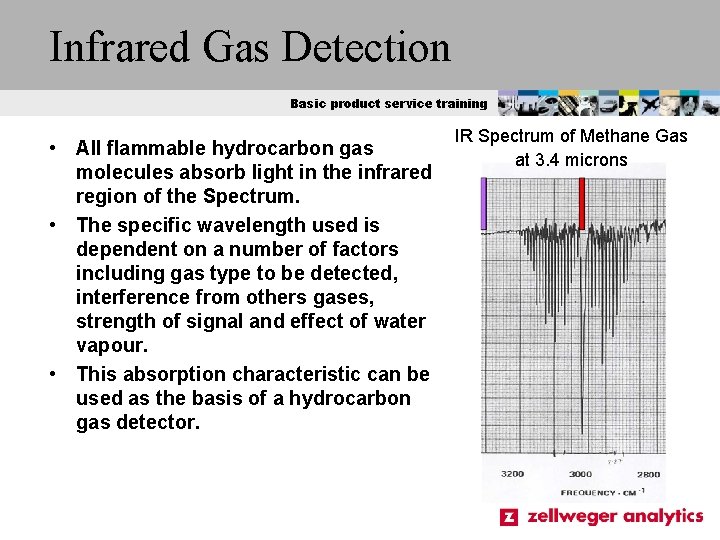
Infrared Gas Detection Basic product service training • All flammable hydrocarbon gas molecules absorb light in the infrared region of the Spectrum. • The specific wavelength used is dependent on a number of factors including gas type to be detected, interference from others gases, strength of signal and effect of water vapour. • This absorption characteristic can be used as the basis of a hydrocarbon gas detector. IR Spectrum of Methane Gas at 3. 4 microns

Infrared Gas Detection Basic product service training • Infrared gas detectors compare the amount of light at a wavelength where hydrocarbon gas molecules absorb light with an area of the spectrum where no such absorption occurs. • The absorption wavelength is know as the sample wavelength and the wavelength at which no absorption is expected is known as the reference wavelength. • The measurement made is the change in ratio between the sample and reference signals.

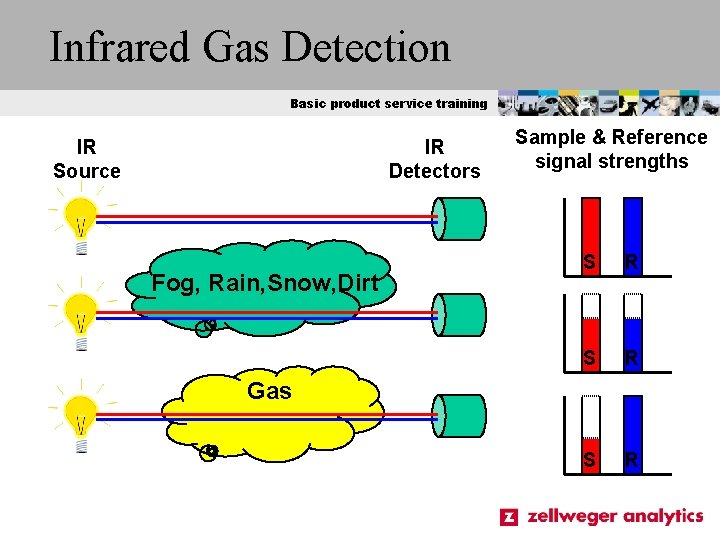
Infrared Gas Detection Basic product service training IR Source IR Detectors Fog, Rain, Snow, Dirt Sample & Reference signal strengths S R S R Gas

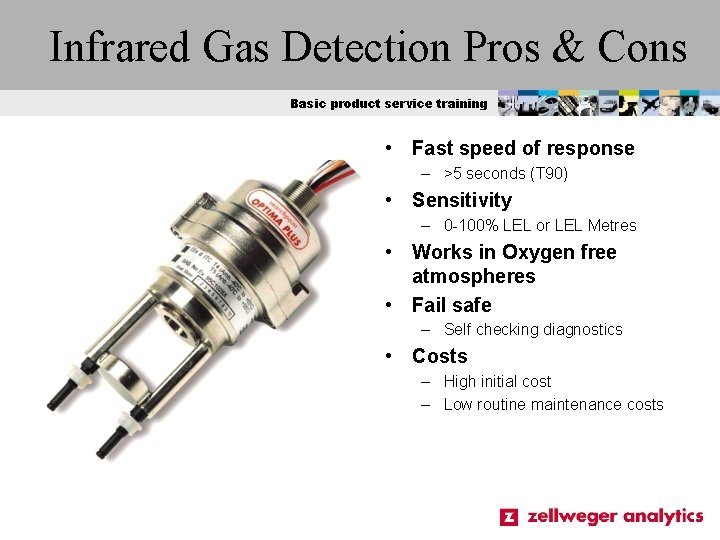
Infrared Gas Detection Pros & Cons Basic product service training • Fast speed of response – >5 seconds (T 90) • Sensitivity – 0 -100% LEL or LEL Metres • Works in Oxygen free atmospheres • Fail safe – Self checking diagnostics • Costs – High initial cost – Low routine maintenance costs

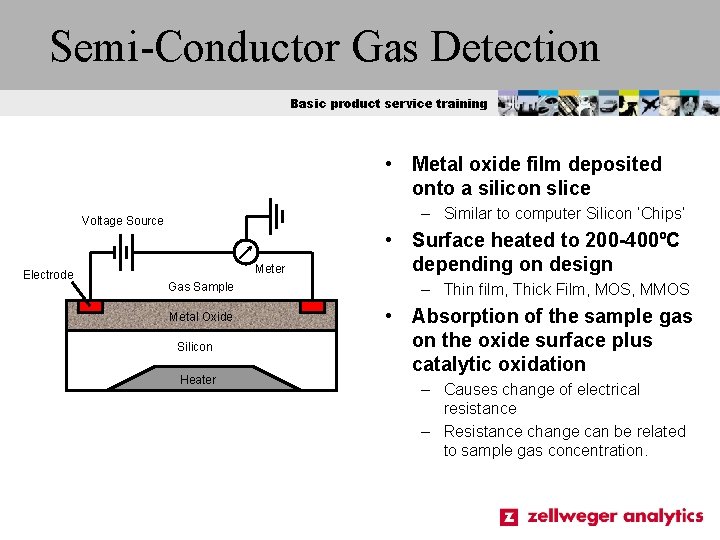
Semi-Conductor Gas Detection Basic product service training • Metal oxide film deposited onto a silicon slice – Similar to computer Silicon ‘Chips’ Voltage Source Electrode Meter Gas Sample Metal Oxide Silicon Heater • Surface heated to 200 -400ºC depending on design – Thin film, Thick Film, MOS, MMOS • Absorption of the sample gas on the oxide surface plus catalytic oxidation – Causes change of electrical resistance – Resistance change can be related to sample gas concentration.

Semi-Conductor Gas Detection Basic product service training • Used in cheap domestic detectors in Japan – High volume low cost • Popular for H 2 S detection – High temp / high humidity applications – Low temp / low humidity applications • Limited range of gases – susceptible to cross interferences from other gases • Non Linear output – Added circuitry/complexity of reading • Susceptible to environmental fluctuations • Some designs can ‘fall asleep’ – Negative signal drift • Frequent gassing often required • Some designs very high power – Higher cost of battery back up • Costs – Low initial cost – High routine maintenance costs

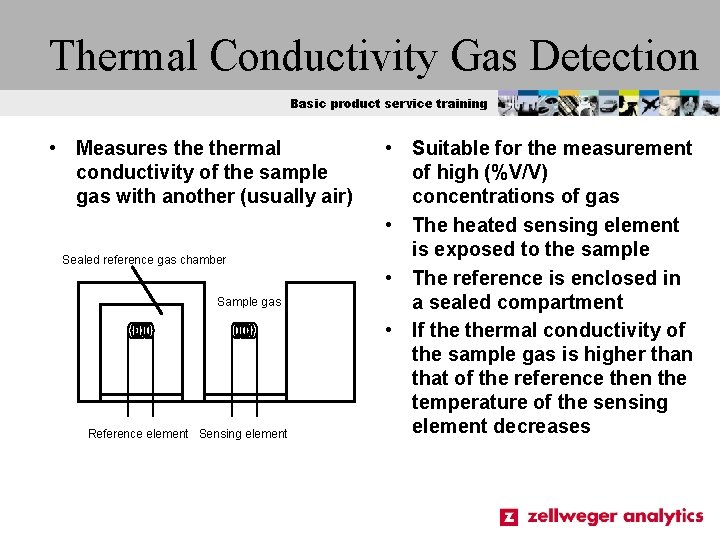
Thermal Conductivity Gas Detection Basic product service training • Measures thermal conductivity of the sample gas with another (usually air) Sealed reference gas chamber Sample gas Reference element Sensing element • Suitable for the measurement of high (%V/V) concentrations of gas • The heated sensing element is exposed to the sample • The reference is enclosed in a sealed compartment • If thermal conductivity of the sample gas is higher than that of the reference then the temperature of the sensing element decreases

Thermal Conductivity Gas Detection Basic product service training • If thermal conductivity of the sample gas is less than that of the reference then the temperature of the sample element increases. • These temperature changes are proportional to the concentration of gas present at the sample element. • Mainly used for detecting gases with a thermal conductivity greater than air – Eg, Methane and Hydrogen • Gases with thermal conductivities close to air cannot be detected. – Eg, Ammonia and CO • Gases with thermal conductivities less than air are more difficult to detect as water vapour can cause interference – E. g Carbon Dioxide and Butane • Mixtures of two gases in the absence of air can also be measured using this technique

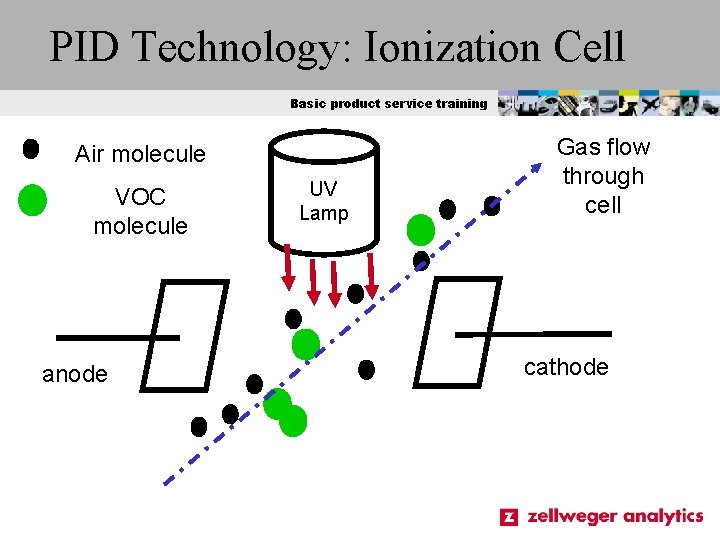
PID Technology: Ionization Cell Basic product service training Air molecule VOC molecule anode UV Lamp Gas flow through cell cathode

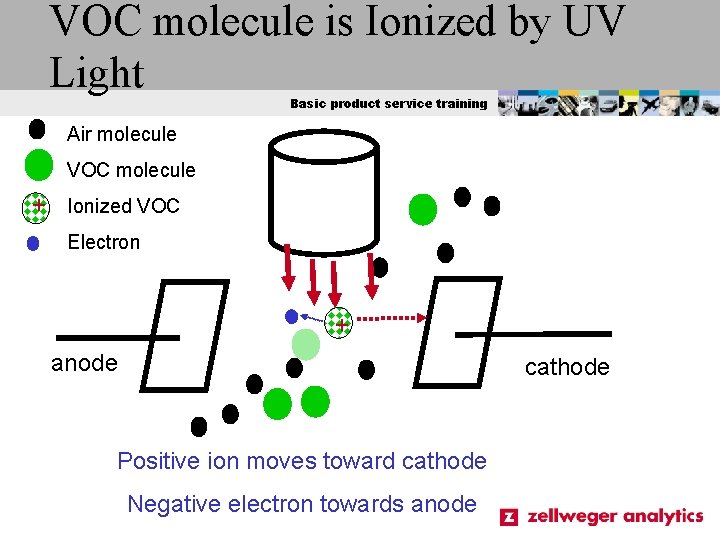
VOC molecule is Ionized by UV Light Basic product service training Air molecule VOC molecule + Ionized VOC Electron + anode cathode Positive ion moves toward cathode Negative electron towards anode

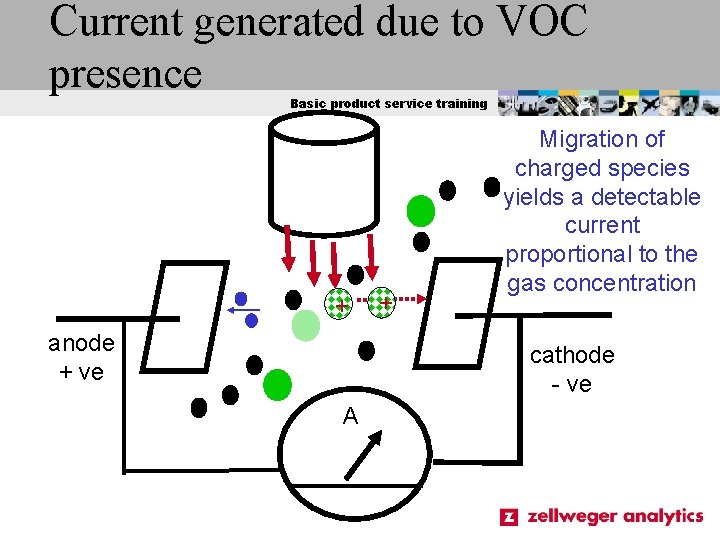
Current generated due to VOC presence Basic product service training + anode + ve + Migration of charged species yields a detectable current proportional to the gas concentration cathode - ve A

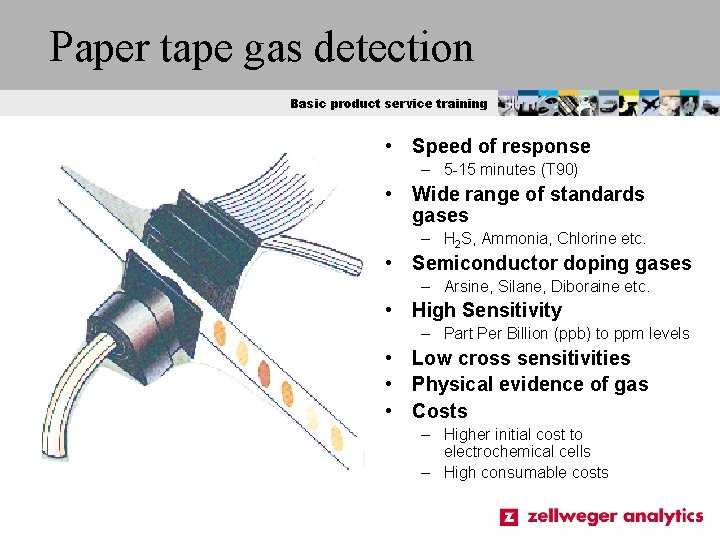
Paper tape gas detection Basic product service training • Speed of response – 5 -15 minutes (T 90) • Wide range of standards gases – H 2 S, Ammonia, Chlorine etc. • Semiconductor doping gases – Arsine, Silane, Diboraine etc. • High Sensitivity – Part Per Billion (ppb) to ppm levels • Low cross sensitivities • Physical evidence of gas • Costs – Higher initial cost to electrochemical cells – High consumable costs

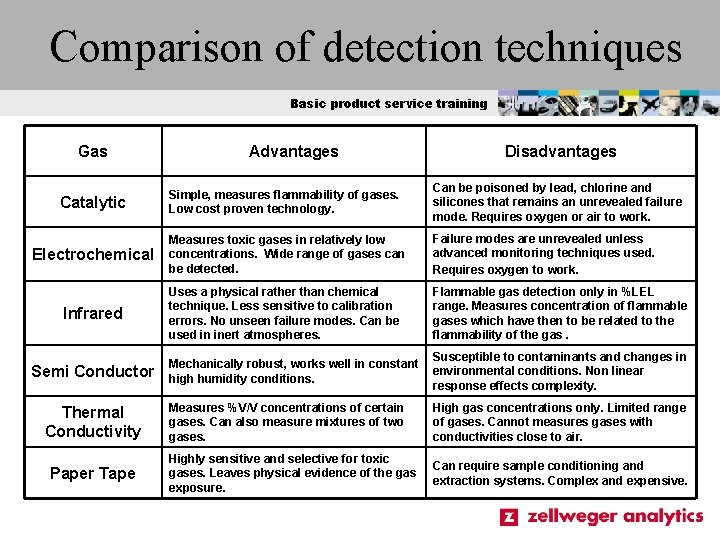
Comparison of detection techniques Basic product service training Gas Advantages Disadvantages Catalytic Simple, measures flammability of gases. Low cost proven technology. Can be poisoned by lead, chlorine and silicones that remains an unrevealed failure mode. Requires oxygen or air to work. Electrochemical Measures toxic gases in relatively low concentrations. Wide range of gases can be detected. Failure modes are unrevealed unless advanced monitoring techniques used. Requires oxygen to work. Infrared Uses a physical rather than chemical technique. Less sensitive to calibration errors. No unseen failure modes. Can be used in inert atmospheres. Flammable gas detection only in %LEL range. Measures concentration of flammable gases which have then to be related to the flammability of the gas. Mechanically robust, works well in constant high humidity conditions. Susceptible to contaminants and changes in environmental conditions. Non linear response effects complexity. Measures %V/V concentrations of certain gases. Can also measure mixtures of two gases. High gas concentrations only. Limited range of gases. Cannot measures gases with conductivities close to air. Highly sensitive and selective for toxic gases. Leaves physical evidence of the gas exposure. Can require sample conditioning and extraction systems. Complex and expensive. Semi Conductor Thermal Conductivity Paper Tape